Abstract
Background: New therapies for AML are urgently needed. IMGN779 is a novel CD33-targeting ADC that utilizes as the cytotoxic agent a DNA-alkylatingindolinobenzodiazepine, DGN462. Previous data have shown that IMGN779 exerts dose-dependent activity against human CD33+ AML cells in vitro and in vivo. We hypothesized that combination treatment of AML cells with the poly (ADP-ribose) polymerase (PARP) inhibitor, olaparib, which blocks cellular DNA repair would further enhance the anti-leukemic activity of IMGN779 in preclinical human AML models.
Methods:Human CD33+ AML cell lines (HEL, MV4-11, HL60) were treated in vitro with controls, IMGN779, olaparib, and IMGN779 + olaparib. Proliferation was measured by WST-8 reagent. Synergistic/additive effects were calculated using Compusyn software. Flow cytometry was performed to assess apoptosis, viability, and cell cycle effects. SCID mice were engrafted systemically with human AML (HEL-luciferase) cells followed by treatment with IMGN779, olaparib, or both drugs in combination. Changes in disease burden and possible treatment-related toxicities were determined by whole animal bioluminescent imaging, body weights, and time to morbidity, respectively. Primary cells from patients with relapsed/refractory AML characterized by complex karyotype or FLT-3 mutations were plated in methocellulose media supplemented with hematopoietic cytokines for colony formation unit assays (CFU). Vehicle, IMGN779 and/or olaparib in triplicate were added followed by quantification of leukemic CFU 15 days later with a Spot-RT3 camera mounted to an inverted microscope.
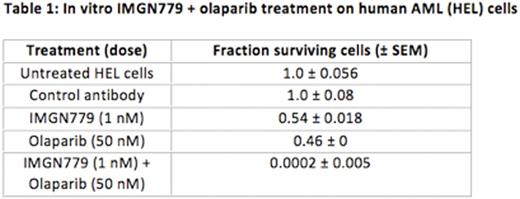
Results: IMGN779 treatment induced significant growth inhibition in vitro in all CD33+ human AML cell lines tested that was dose dependent. IMGN779 cell killing was CD33 dependent. Olaparib induced cell death in human AML cell lines via reversal of DNA damage repair mechanisms. Combination treatment with IMGN779 (500 pM-1 nM) and olaparib (10-50 μM) significantly enhanced anti-leukemic effects over monotherapy in the same cell lines (representative data in Table 1). Combination indices for IMGN779 and olaparib therapy ranged from 0.7-0.9, consistent with synergistic effects. The combination markedly reduced overall cell viability, increased apoptosis, and induced almost complete S-phase cell cycle arrest as compared with controls and single-agent treatments. Exposure to a combination of IMGN779 and olaparib also significantly inhibited CFU growth of progenitor cells established from bone marrow samples of patients (n=7) with relapsed/refractory, FLT3-ITD, and/or complex karyotype AML. Statistically significant inhibition of viable CFUs was observed following combination IMGN779 (10 pM) and olaparib (1 μM) therapy as compared with monotherapy or vehicle controls (p<0.001).
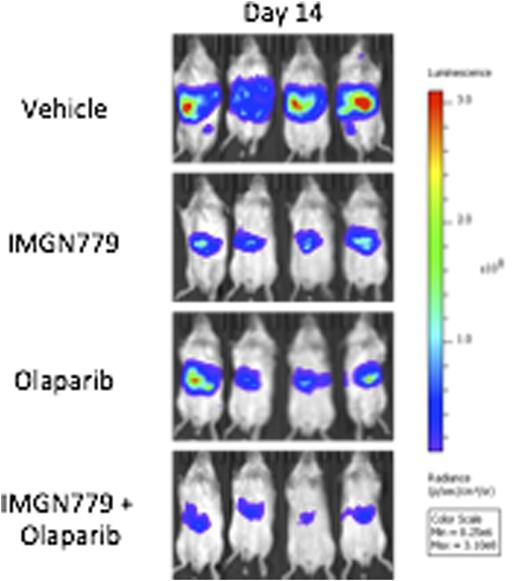
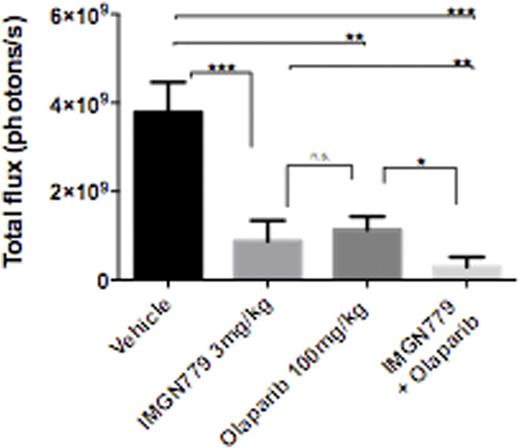
In vivo, IMGN779 administered as a single dose that ranged from 0.5 to 5 mg/kg, by antibody, was overall well tolerated in SCID mice bearing systemic human CD33+ AML (HEL-luciferase) xenografts. Significant dose-dependent anti-leukemic activity, as reflected by decreased leukemia burden and prolonged overall survival, was observed. Combination treatment with IMGN779 (3 mg/kg) and olaparib (100 mg/kg) further significantly decreased leukemic burden when compared with vehicle (p<0.0001), IMGN779 alone (p<0.01) and olaparib alone (p<0.05) on day 14 after dosing (Figure 1).
Conclusion:The combination of the CD33-targeting ADC, IMGN779, and the PARP inhibitor, olaparib, enhanced anti-tumor activity in multiple preclinical human CD33+ AML models. The combination increased in vitro DNA damage, apoptosis, S-phase arrest, and cell death effects on human CD33+ AML cells vs. single agent therapy. Combination therapy also markedly inhibited colony formation of primary AML cells representing clinically chemoresistant disease and significantly decreased in vivo leukemic burden in systemic human AML xenograft models. Our results support the future clinical investigation of this novel combinatorial regimen for AML therapy.
In vivo IMGN779 + olaparib treatment in systemic HEL AML xenografts
In vivo IMGN779 + olaparib treatment in systemic HEL AML xenografts
Walker:ImmunoGen, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.