Abstract
Introduction: AML is the second most of pediatric leukemia with relapse in >30% of the patients.Clonal evolution of rare primary leukemic cells that survived the initial therapy or gained additional mutations independent of therapy stress, could be the potential cause of relapse in pediatric AML.Therefore, sensitive and reliable methods to measure accurately the mutational shifts between diagnoses, during therapy and relapse could provide useful information on the disease progression.Exosomes are extracellular vesicles of 30-150nm in diameter that are released by both healthy and malignant cells. Exosomes derived from tumor cells or leukemia blasts have emerged as potential valuable biomarkers as they have been illustrated to feature disease specific protein, lipid and nucleic acid signatures that represent the pathological state of the respective cells. While leukemia cells release relatively higher amounts of exosomes compared to healthy cells, mutational profiling of exosomes could be more sensitive than analyzing rare leukemia sub-clone in thehematopoietic compartment.
Methods: Secreted exosomes from conditioned media of K-562leukemia celllines and plasma of pediatric AML patients were isolated using differential ultracentrifugation at 100000g. Absolute DNA amount was quantified usingQuantiFluordsDNA System fromPromega. DNA deep sequencing was performed to analyze theexosomal DNA from K-562 cell line. The mutational profiling of the DNA from primary material, plasma and exosomes was performed using next generation sequencing platform, Illumina.
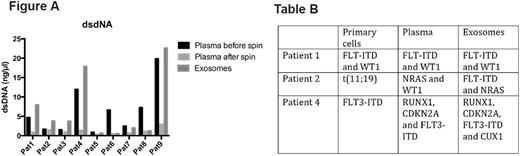
Results: To establish the diagnostic and prognostic potential of exosomes in measuring leukemia associated mutations we isolated exosomes from the conditioned media of the cultured leukemia cell lineK-562. We used this cell line as model because it harbors the classical BCR-ABL translocation that is relevant in deciding treatment options in chronic myeloid leukemia. Analysis of DNA isolated from these exosomes revealed the presence of genomic double-stranded DNA (dsDNA) fragments. Using deep sequencing approach we could further detect the classical BCR-ABL translocation in exosomes. The presence of leukemia specific mutation in exosomes that is potentially derived from parental cells suggests the utility for exosomes in leukemia diagnosis and to forecast treatment response and relapse. In the next step, we analyzedexosomal dsDNA from plasma exosomes of primary pediatric AML patients. We compared the sensitivity of DNA associated from exosomes isolated from blood plasma of pediatric AML patients and compared it to free floating cell free DNA (cfDNA) in the plasma supernatant. In our study we used the plasma volume ranging from 350 microliters to 1ml from peripheral blood of AML patients at diagnosis and relapse (n=9). We performed DNA isolation from the starting plasma material and then compared it with the exosomes isolated. As control, DNA from the exosomes depleted fraction was also analyzed. Comparison of these three fractions (input plasma, pelleted exosomes and exosome depleted plasma) revealed a significant enrichment of dsDNA in the exosome fraction after ultracentrifugation in 7 out of 9 patients (Figure A). This suggests that isolation of exosomes from supernatant can enrich the amount of DNA and thereby the sensitivity of downstream mutational analysis in diagnostic. By next generation sequencing (NGS) (myeloid panel, Illumina) using the DNA materials from plasma supernatants and the respective exosomes we screened for AML associated mutations (n=7). We were able to detect the known primary mutation detected at diagnosis or relapse in both plasma and exosomes of 6 out of 7 patient material. In one patient the detection of mutations were not possible in both the plasma andexosomal fraction. Interestingly we could detect additional mutations like FLT-ITD (patient 2) or CUX1 (patient 4) only in the exosome fraction (Table B).
Conclusion: These results suggest that the combined evaluation of DNA from plasma and exosome fraction could provide novel and additional information about the clonal hierarchy or evolution of AML, and if used for monitoring, might be of prognostic relevance.
Reinhardt:Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees; Jazz Pharma: Other: Travel Accomodation; Celgene: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.