Abstract
Introduction:
Adult T-cell leukemia/lymphoma (ATL) is highly aggressive malignancy caused by human T-cell leukemia virus type 1 (HTLV-1). Because ATL cells are very aggressive, cytotoxic combination chemotherapy is used as first-line therapy. However, ATL cells are often resistant to chemotherapy. The intensive chemotherapy induces severe myelosuppression, which requires frequent transfusions, and organ damages. Unfortunately, accumulation of these adverse events disables continuation of treatment in some patients. Recently antibody therapy which targets CC chemokine receptor 4 (CCR4) expressed on ATL cells has been approved for the treatment of relapsed ATL, but lymphoma-like massive ATL cells in lymph nodes or spleen are known to be resistant to antibody therapy. Therefore, we sought to develop a new strategy, and finally focused on amino acids as asparaginase is used for treatment of acute lymphoblastic leukemia.
Methods:
Amino acid dependency of HTLV-1-infected cells, including ATL cells, was examined in vitro using HTLV-1-infected cell-lines and ATL cells which were serially passaged after purification by flow cytometry from the sample of an acute-type ATL patient. These cells were cultured with stromal cells in the medium which lacked one of twenty amino acids for 2 weeks. Proliferation of ATL cells and influence on stromal cells was examined, and we picked up amino acids which were crucial only for ATL cells. We next prepared diets which lacked specific amino acids based on in-vitro screening, and examined the effect in vivo using xeno-transplantation models. In a lymphoma model, massed ATL cells were transplanted intraperitoneally into adult NOG mice, which were fed complete diets. Diets were unchanged or changed to restricted ones after 6 weeks, and maximum tumor size was compared after 10 weeks. In a leukemia model, primary ATL cells, which were sorted from peripheral blood of an acute-type ATL patient, were transplanted intravenously into neonatal NOG mice within 24 hours from the birth. Recipient mice were brought up by the mother fed complete diets. After 4 weeks, recipient mice were weaned from breast, and fed complete diets or restricted ones. Peripheral blood was followed over time by flow cytometry, and infiltration of ATL cells into various organs was analyzed after 8 weeks.
Results:
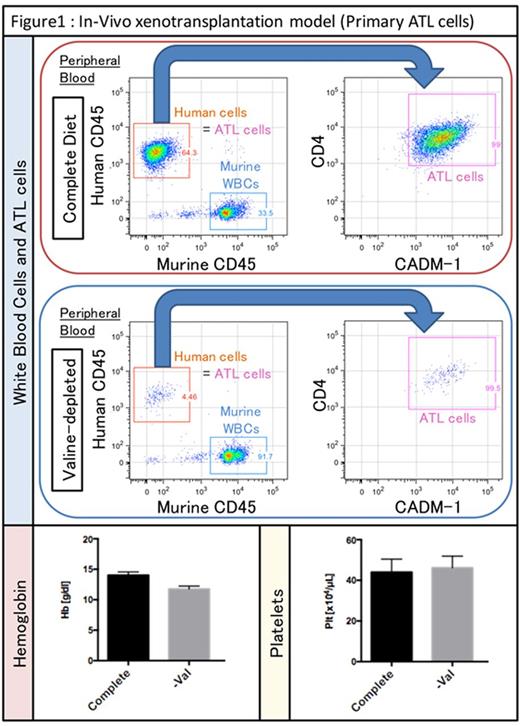
In-vitro studies revealed that HTLV-1-infected cells have dependency on specific amino acids in common, and it was noteworthy that ATL cells could not proliferate in valine-deficient conditions while the influence on co-cultured stromal cells was limited. Therefore, we specially prepared valine-depleted diets, and confirmed that density of valine was very low in peripheral blood and bone marrow of mice fed valine-depleted diets. In-vivo lymphoma models showed that no masses were observed macroscopically in all of mice fed valine-depleted diets. In-vivo systemic leukemia models showed that valine-restricted diets preferentially reduced ATL cells but not hemoglobin or platelets in peripheral blood as shown in figure 1. Additionally, pathological examinations showed that valine-deficient diets strongly prevented ATL cells from proliferating and infiltrating into organs, such as spleen, liver, and skin. As far as we examined histologically, there were no significant organ damages.
Discussion and Conclusions:
Our study uncovered that adult T-cell leukemia/lymphoma cells failed to proliferate in valine-depleted conditions. The inhibition induced by dietary valine restriction was drastic, whereas ATL cells could proliferate to greater or lesser degrees in conditions lacking other amino acids, even if essential amino acids were depleted. Additionally, valine is indispensable for the proliferation and maintenance of hematopoietic stem cells (HSCs) as we previously reported. Therefore, valine-deficient diets could successfully reduce leukemic stem cells of ATL. Moreover, there were no severe complications such as anemia, thrombocytopenia, and organ damages which are often seen in chemotherapy recipients. Lymphoma-like massive ATL cells, which are often resistant to antibody therapies, were also vulnerable to valine-depleted conditions. The issues seen in currently available therapies against aggressive leukemia and lymphoma could be solved by this diet therapy itself or by adding this therapy to other treatments with reduced toxicity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.