Abstract
The bone marrow (BM) microenvironment contains numerous cell types, including immunosuppressive tumor-associated macrophages (TAM), that promote the growth and survival of multiple myeloma (MM) cells. TAMs are recruited to the site of MM within the BM and help to create a permissive microenvironment that promotes the homing of MM cells. Prior studies have shown that the relative amount of BM-associated TAMs correlates with patient poor outcome in MM (Suyaný et al. 2013). Emerging evidence suggests that the absolute monocyte count (AMC) in peripheral blood may reflect the level of TAM recruitment at the tumor site and its prognostic significance has been shown in other lymphoid malignancies. Absolute lymphocyte count (ALC) as a surrogate marker for host immune system has prognostic significance. Early lymphocyte recovery after induction therapy or autologous stem cell transplant correlates with better survival for MM patients. The aim of the present study was to investigate the prognostic significance of the ratio of ALC, as a surrogate of host immune status, to AMC, which reflects the MM-immune microenvironment, in predicting clinical outcome among newly diagnosed MM patients.
Methods: The study included 372 MM patients (pts), diagnosed 2004-2014 at the University Hospitals in Cleveland, OH and University of Cincinnati, Cleveland OH who had CBC differentials at diagnosis. The primary end-point of the study was progression free survival (PFS) calculated from the time of diagnosis. The Cox proportional hazards model was used to evaluate ALC/AMC at diagnosis as a prognostic factor for PFS as well as to assess and adjust of other know prognostic factors.
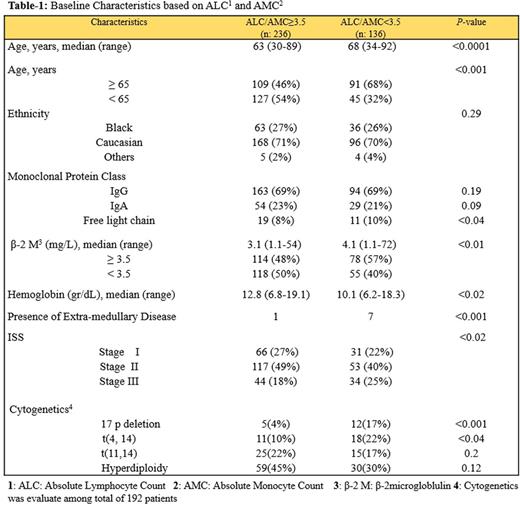
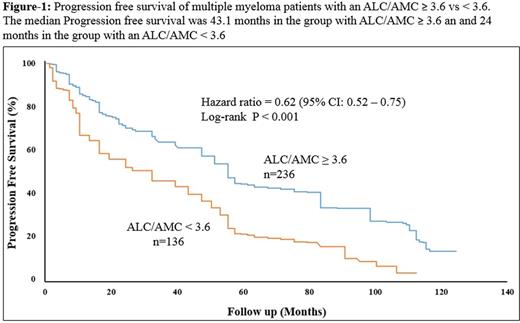
Results: Median pt age was 67.3 years (range: 30-92), 53% were male and 47% female. Distributions of detailed baseline characteristics are presented in Table-1. The median follow up was 33.5 months (range: 1.16 - 122.9). Pts who were lost to follow up were censored from the survival analysis. At the time of this analysis 92 pts (24%) had died, 75 (20%) of them was due MM. The median AMC, ALC and ALC/AMC ratio at diagnosis was 412 cells/µL, 1,461 cells/µL and 3.9, respectively. ALC neither AMC was associate with different PFS or overall survival (OS). ALC/AMC as a continuous variable was identified as a predictor of PFS [Hazard ratio (HR) = 0.62, (95% CI: 0.52 - 0.75), p-value: 0.001]. To define a cut-off point, the choice of ALC/AMC ≥ 3.6 yielded the greatest differential in survival, based on the chai-squarevalue (χ2 = 94·4, P < 0·01) analyzed at different cut-off points between the 25% and 75% quartiles (2.9 - 4.3), from the log-rank test. Pts with an ALC/AMC ≥ 3.6 (n=236) experienced a superior median PFS compared to patients with an ALC/AMC < 3.6 (n=136) [Median PFS, 24 vs. 43 months respectively, P-value < 0.001] (Figure-1). Multivariate analysis including ALC, age, ISS stage and treatment type (autologous stem cell transplant vs. no transplant) showed that ALC/AMC as well as treatment type remained an independent prognostic factor of PFS after the step-wise fashion analysis. Out of 372 patients 192 patients had cytogenetics analysis at diagnosis. Univariate analysis demonstrated that low ALC, high AMC and a low ALC/AMC ratio were correlated with presence of del (17p) and t(4,14) and light chain myeloma. Multivariate analysis indicated that the ALC and AMC were not associated with any specific cytogenetic group. However, the ALC/AMC ratio independently predict 17p and t(4,14) abnormalities (p-value = .041). Low ALC, high AMC, and low ALC/AMC ratio were associated with poor prognostic factors such as high β2-microglobulin.
Conclusions: In our cohort, myeloma patients with a higher ALC/AMC at diagnosis enjoyed a longer PFS (either as a continuous or dichotomized variable). Also, multivariate analysis demonstrated that patient with tumors that harbored del (17p) or t(4, 14) had low ALC/AMC ratio suggesting possible immunoparesis. ALC at diagnosis was not associated with OS, in contrast to prior studies, possibly due to the shorter follow-up duration used here and inadequate power to predict OS. Taken together, our data potentially suggest the ALC/AMC ratio as a readily available surrogate marker to assess the relative strength of host immune system to myeloma-induced immunoparesis. The ratio can serve as a biomarker to stratify patients for future immunological therapies. Also, our findings indicate that the host immune status should be carefully monitored.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.