Abstract
Background: Tyrosine kinase inhibitor (TKI) remains to be the mainstay of treatment for patients with chronic myelogenous leukemia (CML). Second generation TKIs have been shown to successfully treat patients who are resistant or intolerant to imatinib, as well as induce faster and deeper molecular response when used as first line therapy. Since the initial report of the French STIM study (Mahon FX, et al. Lancet Oncol 2010; 11: 1029-35) that showed successful discontinuation of imatinib therapy in approximately 50% of patients who have sustained complete molecular response (CMR), several groups have confirmed that a subset of patients can discontinue TKI for a long period. While factors associated with successful discontinuation are not yet well defined, it also remains an open question whether patients who have failed the initial attempt of discontinuation will have to remain on life-long treatment with TKIs.
Patients and Methods: Patients who have been treated at Keio University Hospital for CML, who had been in confirmed stable CMR for over 2 years at the time of study enrollment, and who had no history of accelerated phase/ blastic phase while on treatment with TKI, were eligible to enroll in the study. Patients were monitored monthly for the first 6 months after discontinuation, every 2 months until 12 months, and every 2 to 3 months thereafter. Treatment with a TKI was initiated if the peripheral blood quantitative PCR (TMA method) value exceeded 100 copies. Once the patient was restarted on TKI therapy, and regained sustained CMR for over 2 years, they were allowed to reenter the study and discontinue treatment, upon patients' choice.
Results: Sixty-seven patients have been enrolled in the study, of which 53 patients who have been observed for over 2 years since first TKI (imatinib 48; dasatinib 1; nilotinib 4) discontinuation were analyzed. The median age of the patients was 54 (range 28-83) years. Thirty-seven (69.8%) patients were male. In terms of baseline characteristics, 18 (34.0%) had been treated with interferon prior to TKI use, and 41 (77.4%) were CMV positive. The Sokal risk score was low in 34 (64.2%), intermediate in 11 (20.8%) and high in 4 (7.5%) patients. Among the 53 patients, 45 (84.9%) were checked for the existence of BIM deletion, among which 7 (13.2%) patients were positive. The median time on TKI treatment was 98 (range 32-147) months and the median duration of CMR was 38 (range 24-106) months.
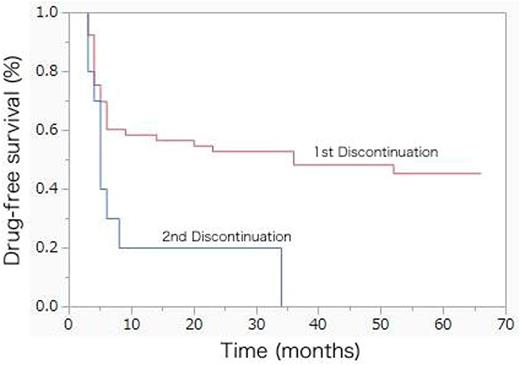
The median follow-up of the patients at the time of this analysis since study enrollment was 61 (range 26-66) months. Treatment was restarted in 28 (45%) patients (imatinib 7; dasatinib 20; nilotinib 1). While this occurred within the first 6 months of treatment discontinuation in most patients, 6 patients were started on treatment beyond 12 months of drug-free survival (DFS) (at month 14, 20, 23, 36, 36, and 52, respectively). Five patients presented with a fluctuating copy number early after TKI discontinuation, whereas 1 patient only became positive for bcr-abl after 30 months of treatment discontinuation. The estimated 24-months DFS was 52.8% (95% confidence interval (CI) 39.5-65.8%) (Fig 1). All patients have restored CMR at least at one occasion after recommencing TKIs. No single factor was significantly associated with success of discontinuation.
Among the patients who had sustained CMR for over 24 months after re-initiation of TKI, 10 patients elected to challenge discontinuation of TKI for the second time. All patients were on dasatinib at the time of discontinuation. The median age of these patients was 58.5 (range 31-75) years. The median time on TKI prior to second discontinuation was 33 (range 26-45) months and the median duration of CMR after treatment re-initiation was 26.5 (range 25-44) months. All but one patient were restarted on treatment at the time of the analysis (median observation 26 (range 13-35) months), leading to a DFS of 20% (95% CI 5.0-54.1%) at 12 and 24 months (Fig 1).
Conclusion: Long-term observation of the outcome of TKI discontinuation in CML patients who had sustained CMR for over 2 years showed cases of late relapses as well as small chance of success on the second attempt of TKI discontinuation even with the use of second generation TKIs. While the result of first discontinuation was similar to previous reports, attempt of second discontinuation was less successful compared to the French group, despite changing the drug of use from imatinib to dasatinib.
Matsuki:Nippon Shinyaku: Honoraria; Celgene: Honoraria; Bristol-Myers Squibb: Honoraria. Sakurai:Celgene: Honoraria. Karigane:Celgene: Honoraria. Kikuchi:Celgene: Honoraria; Takeda Pharmaceutical Company: Honoraria; Kyowa Hakko Kirin: Honoraria. Yokoyama:BMS: Research Funding. Okamoto:Eisai Co., Ltd.: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding; Nippon Shinyaku Co., Ltd.: Research Funding; Alexion Pharmaceuticals, Inc.: Research Funding; Astellas Pharma Inc.: Research Funding; Toyama Chemical Co., Ltd.: Research Funding; Otsuka Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Shionogi & Co., Ltd.: Research Funding; Bristol-Myers Squibb K.K.: Honoraria, Research Funding; Asahi Kasei Pharma Corp.: Research Funding; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; Teijin Pharma Limited: Research Funding; Pfizer Inc.: Honoraria, Research Funding; JCR Pharmaceuticals Co., Ltd.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract