Abstract
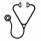
Background Nilotinib (NIL) is a second-generation tyrosine kinase inhibitor (TKI) that exhibits significant efficacy as first- or second-line treatment in patients with chronic myeloid leukemia (CML). Superior rates of deeper molecular responses (DMR) were achieved with NIL vs. imatinib (IM) in patients newly diagnosed with CML in chronic phase (CML-CP) in the ENESTnd trial. In addition, the ENESTcmr study demonstrated that switching to NIL after a minimum of 2 years on IM led to increased rates of DMR vs. remaining on IM. Switching to NIL treatment for 2 years safely led to MR4,5 (BCR-ABLIS…0.0032%) in 47.5% of patients with major molecular response (MMR) on long-term IM therapy in our STAT1 trial. Recently, treatment free remission (TFR) was proposed as one of the goals in CML treatment. Indeed, prospective trials suggest that IM therapy may be safely and successfully discontinued in 40% of CML patients with MR4.5. STAT2 is the first study to evaluate the efficacy of two-year consolidation by NIL for successful TFR in patients with CML-CP who had achieved MR4.5. Before enrolling in STAT2, some patients were treated by not only IM but also NIL because of MMR but no MR4.5 after IM therapy, and some patients changed over from STAT1 to STAT2. Here, we present the results of the subgroup analysis from STAT2 based on the prior treatments at the time of entry into the study.
Methods In the STAT2 trial, patients who achieved MR4.5 on IM front line therapy (subgroup 1; SG1) or NIL second line therapy after IM therapy (subgroup 2; SG2) were eligible and NIL was given twice daily at the dose of 600 mg/day for 2 years in consolidation phase. The primary endpoint of STAT2 was the proportion of patients with successful TFR, defined as no confirmed loss of MR4.5 (2 consecutive IS RQ-PCR tests), within the first 12 months of TFR phase. Thirty-five institutions in STAT study group participated. The study was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was signed by all patients according to institutional guidelines. The study was approved by all institutional review boards and registered with UMIN-CTR (000005904).
Results Between July 2011 and December 2012, 96 patients were enrolled in STAT2. Among 96 patients, 50 patients were treated by IM first line only as prior therapy (SG1). On the other hand, 40 patients were treated by IM first line and NIL second line including 21 patients who changed over from STAT1 to STAT2 because they achieved MR4.5 (SG2). Six patients were excluded in this analysis because second generation TKIs were taken as a first line therapy. Among patients treated by NIL for 2 years in this study, 40/50 (80%; 95% CI, 68.4%-88.7%) in SG1 and 33/40 (82.5%; 95% CI, 69.6%-91.5%) in SG2 entered the TFR phase, respectively. The median age was 54.5 years in SG1 and 56.0 years in SG2. The ratio of men to women was 26:14 in SG1 and 18:15 in SG2. The total duration of TKI treatment was 110 months for the SG1 with a median of 86 months of IM, and 24 months of NIL, and 93 months in SG2 with a median of 62 months of IM, and 31 months of NIL,, respectively. All patients achieved MR4.5 at the time of entry into the study and the median time to MR4.5 was 47 months in SG1 and 60 months in SG2.The proportion of patients who maintained TFR at 12 months after stopping NIL was similar across the 2 subgroups: 25/40 (62.5%; 95% CI, 48.3%-77.3%) in SG1, and 23/33 (69.7%; 95% CI, 54.0%-82.5%) in SG2. The Kaplan-Meier (KM) analysis of TFR survival showed that in the 2 subgroups, the majority of events occurred within the first 6 months after stopping NIL (Figure 1). There were no significant differences between these 2 subgroups.
Conclusion After two-year consolidation by NIL of CML-CP patients who achieved MR4.5, the TFR rate was 67.9% (90%CI: 58.2% to 76.6%) at 12 months in the STAT2 trial. In the present analysis looking at the prior TKI exposure, the TFR rate was similar in patients treated with IM first line only or who switched from IM to NIL before entering the study, despite the fact that the treatment duration of switched patients was slightly shorter. These findings suggest that two-year consolidation by NIL is associated with successful TFR in CML with MR4.5 that was achieved with IM alone or after switching to NIL.
Kaplan-Meiercurve of TFR survival in the 2 subgroups based onthe prior treatmentsbefore two-year consolidation by NIL, IM first line only as prior therapy (subgroup1) and IM first line and NIL second line (subgroup2).
Kaplan-Meiercurve of TFR survival in the 2 subgroups based onthe prior treatmentsbefore two-year consolidation by NIL, IM first line only as prior therapy (subgroup1) and IM first line and NIL second line (subgroup2).
Takahashi:PFIZER: Honoraria, Research Funding; BMS: Honoraria; NOVARTIS PHARMA: Honoraria, Research Funding. Nakaseko:BMS: Honoraria, Research Funding; PFIZER: Honoraria, Research Funding; NOVARTIS: Honoraria. Nishiwaki:Novartis PHARMA: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract