Abstract
Introduction:
Tyrosine kinase inhibitor (TKI) therapy results in excellent responses in the majority of patients with chronic myeloid leukemia (CML). However, point mutations in the BCR-ABL kinase domain that render the kinase less sensitive to imatinib are observed in approximately 40% of patients who relapse on therapy, but only in a minority of patients with primary resistance. The goal of our study was to determine the mutation patterns and treatment outcomes of patients with imatinib resistance in India.
Methods
The study was performed on a series of 309 CML patients between June 2003 and April 2016 who were tested for imatinib resistance as defined by primary or secondary imatinib failure. Mutation analysis on these patients was done upon treatment failure as per the definitions of the European LeukemiaNet. Survival estimates were calculated using Kaplan-Meier product-limit method. Overall survival was defined based from both time of imatinib failure and time of first detection of mutation to date of death or last follow-up. Direct sequencing of the BCR-ABL transcript by the Sanger method was used for IRMA (Imatinib Resistance Mutation Analysis) testing.
Results
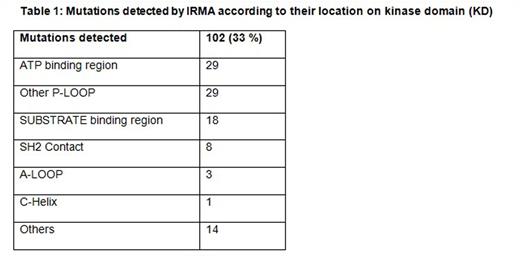
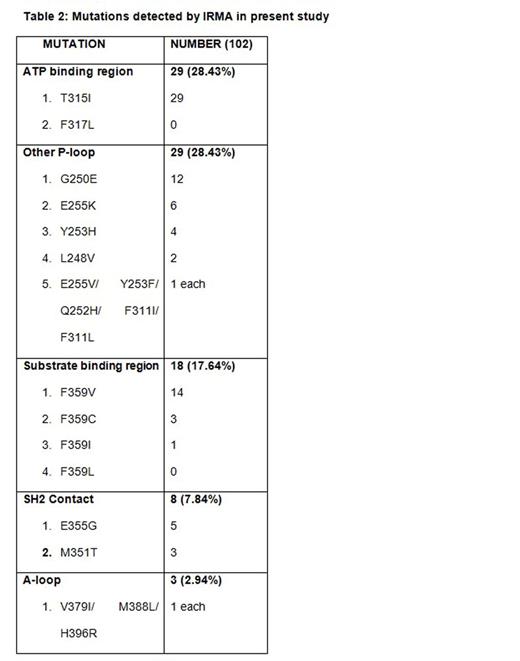
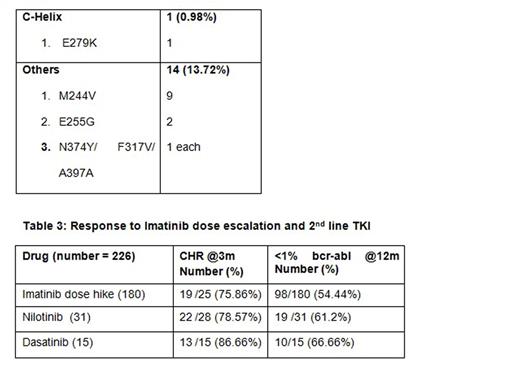
309 patients failing imatinib therapy who were screened for mutations were included in the study. The study group comprised of 67% (208) Male; median age of the study population was 38 years (Range 18 - 66 years) and the median duration of Imatinib usage until the resistance was 24months (Range 3 - 60 months). Of the 309 patients 42% (129) had primary failure and 58% (180) had secondary failure. 33% (102) of the study population had mutations detected of which 18 patients (18%) had primary failure and 84 patients (82%) had secondary failure to imatinib therapy. Specific mutations are detailed in table 1 and 2. Among the 309 patients a total of 226 patients underwent subsequent treatment. Among these 226 patients 180 patients underwent Imatinib dose hike and 31 patients had second line TKI with Nilotinib and 15 patients with Dasatinib. 98 patients (54%) in the Imatinib dose hike group had <1% BCR-ABL at 12months compared to 19 patients (61%) in the Nilotinib and 10(66.6%) in the Dasatinib group. Hematological response in these groups was assessed at 3months and Dasatinib induced notable response with 86% compared to 78.5% in the Nilotinib and 75.8% in the Imatinib dose hike groups (Table 3). Hematologic adverse events were generally mild to moderate in the Dasatinib and Nilotinib groups compared to Imatinib dose escalation, and most cytopenias were effectively managed with dose modifications. On survival analysis (after Imatinib resistance) the 2 year and 4-year survival for the whole group was 91.9% and 69.9% respectively. After a median follow-up of 48 months (Since Imatinib Resistance) the Median overall survival among patients with T315I mutation group (n=29) was 20 months and it was not achieved in those with non T315I mutations (n= 73 )
Conclusion:
BCR-ABL1 kinase domain point mutations were detected in 33% of patients with treatment failure and progression. More than 50% of patients had optimal response to 2nd line TKI and Imatinib dose hike. Presence of the T315I mutation impairs overall survival.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.