Abstract

Objectives Explore co-morbidity profile of persons with chronic myeloid leukemia (CML) in chronic phase receiving tyrosine kinase-inhibitor (TKI) therapy. Identify the effect of co-morbidity(ies) on response to TKI-therapy, patient-reported satisfaction with TKI-therapy, adverse impact on patients' work and daily lives and health-related quality-of life (HRQoL).
Methods We distributed an anonymous, non-interventional, cross-sectional multiple choice questionnaire to ascertain common co-morbidities to adults with CML. Eligibility criteria included persons with chronic phase CML receiving TKI treatment >=3 months. Satisfaction with TKI-therapy was analyzed as: (1) high satisfaction; (2) satisfaction; (3) dissatisfaction; and (4) extreme dissatisfaction. Respondent-reported TKI-therapy-related adverse impact on work and daily life was assessed as Y/N. The SF-36 Health Survey was used to measure HRQOL and used to develop 2 summary scores of physical component summary (PCS) and mental component summary (MCS).
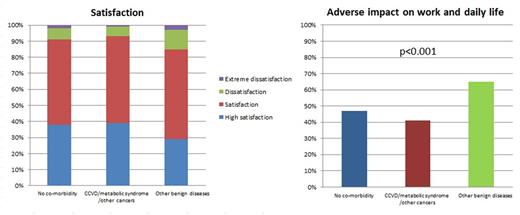
Results Data from 1,143 respondents were analyzed. 725 (63%) were male. Median age was 42 years (range, 18-88 years). 944 (83%) started TKI-therapy <6 months after diagnosis and 868 (76%) were currently on imatinib. 742 (65%) were receiving branded TKIs drugs including Glivec®, Tasigna® and Sprycel®. Median TKI-therapy-duration was 29 months (range, 3-182 months). 878 (78%) achieved a complete cytogenetic response (CCyR) and 479 (43%), a complete molecular response (CMR). 307 (27%) had >=1 co-morbidity and 79 (7%) had >=2 co-morbidities including hypertension (30%), diabetes (21%), heart (12%), gastro-intestinal (12%) liver (12%), kidney (8%), cerebro-vascular (7%) and lung diseases (6%), thrombosis (1%), other cancer (8%) and other (15%). Increasing age (OR=2.0; 95% CI, 1.7, 2.2; p<0.001) and starting TKI treatment >6 months from diagnosis (OR=1.5; [1.0, 2.1]; p=0.040) were associated with co-morbidity(ies) in multivariate analyses. 97 respondents (32%) with co-morbidity(ies) were receiving drugs in addition to TKIs. Respondent satisfaction levels with TKI-therapy were: (1) high satisfaction, 37%; (2) satisfaction, 53%; (3) dissatisfaction, 8%; and (4) extreme dissatisfaction, 2%. 497 respondents (43%) reported TKI-therapy-related negatively impacted work and daily life. There was no significance between type of TKI, achieving CCyR or CMR and respondent satisfaction with TKI-therapy. Co-morbidity profile was significantly-associated with respondent-reported >= satisfaction with TKI-therapy: no co-morbidity, 759 (91%); cardio-cerebral vascular diseases (CCVD), metabolic syndrome and/or other cancers, 176 (93%); and other benign diseases, 99 (85%; p-value for trend=0.056). Co-morbidity profile was also significantly-associated with respondent-reported TKI-therapy-related adverse impact on work and daily life: no co-morbidity, 363 (47%); CCVD, metabolic syndrome and/or other cancers, 68 (41%); and other benign diseases, 66 (65%; p-value for trend <0.001). Co-morbidity profile was also significantly correlated with HRQoL outcomes. PCS score for each co-morbidity profile were: no co-morbidity, 47.9+/-8.7; CCVD, metabolic syndrome and/or other cancers, 44.4+/-9.8; and other benign diseases, 43.2+/-9.9; p<0.001. MCS score for each co-morbidity profile were: no co-morbidity, 49.9+/-10.9; CCVD, metabolic syndrome and/or other cancers, 52.3+/-10.7; and other benign diseases, 46.0+/-11.2; p<0.001. (Figure) Multivariate analyses showed co-morbidity profile was an independent factor affecting patients' work and daily life (OR=1.4; [1.4-1.9]; p=0.008), PCS (OR=1.4; [1.2-1.7]; p=0.001) and MCS (OR=1.5; [1.2-1.9]; p=0.002).
Conclusions The survey showed that 27% (24, 29%) of persons with chronic phase CML receiving TKI-therapy had co-morbidity(ies). CCVD and/or metabolic syndrome were the most common. Co-morbidity(ies) were more common in older subjects. Co-morbidity profile significantly influenced patients' work, daily life and HRQoL. Assessing co-morbidity profile in persons with CML is important to identify persons needing improvement of quality-of-care and HRQoL.
Satisfaction, adverse impact on work and daily life by co-morbidity profile
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract