Abstract
Background
Bone marrow fibrosis (BMF) is observed in 10-20% of patients diagnosed with MDS. A study conducted at the University of Pavia, Italy evaluated 180 cases of MDS with varying grades of BMF and found that the presence of grade 2-3 BMF in MDS (graded by European classification) was associated with worse outcomes, especially in patients with lower risk MDS, however there remains little data pertaining to the treatment and outcomes in the MDS-F patient population. The primary objective of this study is to examine a large MDS patient population to further determine the relationship between BMF and survival outcomes in the context of new risk stratification models.
Methods
All bone marrow aspirate and biopsy reports, obtained at the time of diagnosis or prior to any therapy, in the Moffitt Cancer Center MDS database were reviewed and the degree of BMF was determined using the European classification system. Patients with less than 5% myeloblasts and grade 2 or 3 BMF were identified and these bone marrow aspirate and biopsies were reviewed by two hematopathologists (LZ and JMB) to confirm the grade of fibrosis reported. Patients were then divided into two groups: grade 0-2 BMF and severe BMF (grade 3). These two groups were compared to evaluate differences in clinical characteristics, response to treatment and survival. NexGen sequencing was available for 251 patients and distribution of acquired somatic mutations were compared between grade 0-2 BMF and grade 3 BMF.
Results
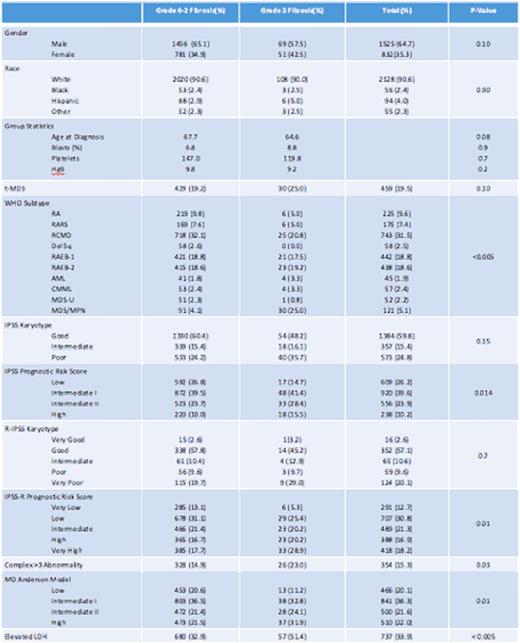
There were 2357 cases included in this analysis of which 2237 (95%) were determined to have grade 0-2 BMF and 120 (5 %) to have grade 3 BMF. Table-1 summarizes baseline characteristics. There was no statistical difference in age at diagnosis, gender, or race. A greater percentage of patients with severe BMF (39%) met the criteria for poor/very poor IPSS-R score category than patients with grade 0-2 BMF (29%), p = 0.011. Complex karyotype was observed more frequently among patients with severe fibrosis (23% versus 15%, p = 0.031). Patients with severe BMF also had a higher incidence of elevated LDH (51%) than patients with grade 0-2 BMF (33%), P < 0.005.
The median overall survival (OS) was 38.7 months (mo) for grade 0-2 BMF and 23.1mo for grade 3 BMF, p < 0.005. When examined by revised IPSS (R-IPSS), severe BMF only impacted OS among the lower risk group. The median OS in patients with very low/low R-IPSS risk was 47mo in patients with severe BMF compared to 77 for those with grade 0-2 BMF, p = 0.015. In multivariable analysis, adjusting for age and R-IPSS, severe BMF was independently associated with inferior OS (HR 1.7, P 0.01). The rate of AML transformation was 28% in both the severe and grade 0-2 BMF groups, p = 0.98.
Eighty patients with severe BMF were treated with hypomethylating agents (HMA). The overall response, by IWG 2006 criteria, of HI or better was 30% among patients with severe BMF compared to 32% of patients with grade 0-2 BMF, p 0.4. Among patients with severe BMF treated with lenalidomide (n=30), 25% of patients had HI response compared to 16% in patients with grade 0-2 BMF, p 0.9. The median OS for the 100 patients with severe BMF who did not undergo allogenic SCT was 23mo compared to 30mo for the 19 patients with severe BMF who did undergo allogenic SCT, p 0.29
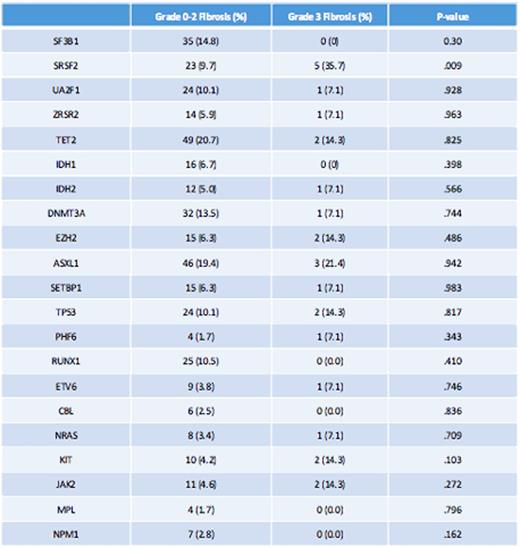
Among somatic gene mutations, SF3B1 mutation was observed in 14% of grade 0-2 and 0% of grade 3 fibrosis. However, SRSF2 was present in 35.7% of patients with grade 3 fibrosis and only 9.7% of patients with grade 0-2 BMF, p .009. There was no statistically significant difference in the rate of occurrence of TP53 and RUNX1 mutations between the grade 0-2 BMF and the grade 3 BMF groups. In addition, there was no significant difference in the rate of occurrence of JAK2 mutation across the two groups. (table-2)
Conclusions
In our MDS cohort, only the presence of severe BMF (grade 3) was associated with worse outcome with reduced overall survival namely among patients with very low/low R-IPSS disease, whereas BMF grade did not impact response to HMA or lenalidomide treatment. SRSF2 gene mutation occurred with greater frequency among patients with severe fibrosis.
Sweet:Incyte Corporation: Research Funding; Ariad: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Pfizer: Speakers Bureau; Karyopharm: Honoraria, Research Funding. Bennett:Celgne: Membership on an entity's Board of Directors or advisory committees. Komrokji:Incyte: Consultancy; Novartis: Consultancy, Speakers Bureau; Boehringer-Ingelheim: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract