Abstract
Overexpression of the antiapoptotic protein Bcl-2 is considered primarily responsible for the increased apoptosis resistance and prolonged survival of the malignant B cells in chronic lymphocytic leukemia (CLL), follicular lymphoma and subsets of diffuse large B cell lymphoma (DLBCL). The importance of Bcl-2 overexpression is corroborated by the significant clinical activity of the recently approved Bcl-2 BH3 antagonist venetoclax. Venetoclax induces apoptosis by sequestering Bcl-2 from the pro-apoptotic Bcl-2 family members Bim, Bax and Bak, resulting in consequent Bax/Bak activation, mitochondrial outer membrane permeabilization and cytochrome c release. Although venetoclax is extremely toxic against CLL cells in vitro, resistance can arise in vivo because of overexpression of other anti-apoptotic Bcl-2 family members, such as Bcl-xL and Mcl-1.
In the present study we investigated the activity of BDA-366, another small molecule Bcl-2 inhibitor that was recently reported to selectively induce apoptosis in Bcl-2-overexpressing lung cancer cells through a distinct mechanism (Han B et al, Cancer Cell. 2015;27:852-63). This drug was designed to selectively bind the BH4 domain of Bcl-2 and was reported to trigger apoptosis by inducing a conformational change in Bcl-2 that converts it into a pro-apoptotic protein. To investigate whether BDA-366 is also selectively toxic for CLL cells, we analyzed the viability of CLL (n=31) and normal peripheral blood mononuclear cells (PBMCs)(n=6) after 48 hours in culture with increasing concentrations of BDA-366. Annexin V/PI analysis showed that BDA-366 is significantly more toxic against CLL (LD50=1.11±0.46 mM) than normal PBMCs (LD50=2.03±0.31 mM, P<0.001). However, considerable variability was noted in the sensitivity of the different CLL samples, with approximatelly 50% of the cases demonstrating LD50 values in the nanomolar range and the remaining cases displaying sensitity similar to normal PBMC. To investigate the reasons for this variability, we correlated LD50 values with IGHV mutation status, ZAP-70 expression, cytogenetic abnormalities, and expression of various Bcl-2 family members, including Bcl-2, Bcl-xL, Mcl-1, BimEL, BimL, Bax, Bak and Hrk. The only positive finding from this analysis was the inverse correlation between LD50 and expression of BimL (n=23, r=-0.446, p=0.033), whereas no correlation was observed with Bcl-2 expression.
Variable sensitivity to BDA-366 and absence of correlation with Bcl-2 expression was also observed in the analysis of a series of DLBCL and CLL cell lines. These cell lines showed LD50 values ranging from nanomolar (Pfeiffer, 0.19 µM; OCI-LY-18, 0.32 µM; OCI-LY-1, 0.33 µM; TOLEDO, 0.41 µM; OCI-Ly10, 0.50 µM; HT, 0.56 µM; Ri-1, 0.58 µM; and SU-DHL-6, 0.81 µM) to micromolar range (MEC1, 1.5 µM; HBL-1, 4.0 µM; Karpas, 4.34 µM; BJAB, 4.8 µM; and SU-DHL-4, 6.31 µM). Although most of the sensitive lines expressed Bcl-2, some had very low or even undetectable Bcl-2 expression (e.g., Pfeiffer and HT), suggesting that BDA-366 can induce apoptosis independently of Bcl-2. In support of this possibility, we observed that BDA-366 was equally effective against Bcl-2-negative HT cells and HT cells stably transfected with Bcl-2. In addition, transient overexpression of Bcl-2 in the DLBCL cell line BJAB and the CLL cell line MEC1 resulted in greater resistance to BDA-366, further suggesting that BDA-366 does not induce apoptosis by converting Bcl-2 into a proapoptotic protein.
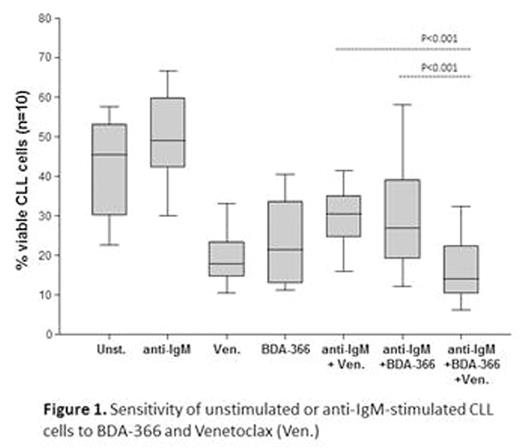
To determine whether mechanisms that induce resistance to venetoclax can also induce resistance to BDA-366, we investigated viability of BDA-366-treated CLL cells following stimulation with immobilized anti-IgM. Pre-treatment of CLL cells with immobilized anti-IgM resulted in increased resistance to both BDA-366 and venetoclax. Interestingly, the combination of BDA-366 and venetoclax was significantly more toxic than each agent alone (Figure 1), suggesting that combining these drugs could be a potential approach to overcome resistance induced by BCR signals.
In conclusion, these data show that BDA-366 is selectively toxic at low nanomolar concentrations for a large proportion of primary CLL samples and DLBCL cell lines. The primary mechanism of action is still unclear, but the lack of a correlation with Bcl-2 expression suggests that it differs from the one initially proposed.
Efremov:Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.