Abstract
Introduction
Immunoglobulin light chain amyloidosis (AL Amyloidosis) is a monoclonal plasma cell proliferative disorder that is characterized by tissue deposits of misfolded insoluble κ or λ light chain derived amyloid fibrils, leading to organ dysfunction. The prognosis of patients depends on the number and severity of organ involvement, especially cardiac involvement. Autologous stem cell transplant (ASCT), if eligible, alkylator (melphalan) and novel drugs like proteasome inhibitors (PI) and immunomodulators (IMiD) have improved the overall survival (OS) during the past decades. But still, nearly half of the patients die within a year of diagnosis. We analyzed the factors predicting early relapse / progression or death (within 12 months) after first line therapy for systemic AL amyloidosis.
Methods
Clinical and laboratory data of all consecutive patients with systemic AL amyloidosis seen at Mayo Clinic within 90 days of their diagnosis, between 2006 and 2015, was collected by chart review and analyzed retrospectively. Patients who died within 3 months of starting the first line treatment were excluded from analysis. Early relapse (ER) was defined as relapse / progression requiring treatment change / re-institution or death within 12 months of starting first line treatment. Patients in the cohort with ER were compared with patients with a follow up of more than 12 months who had a relapse / progression beyond 12 months or had continuing response at the time of analysis. Categorical variables were analyzed using chi - square and Fisher's exact test and continuous variables using Kruskal- Wallis test and Wilcoxon rank sum test. Multivariate analysis was done using logistic regression model.
Results
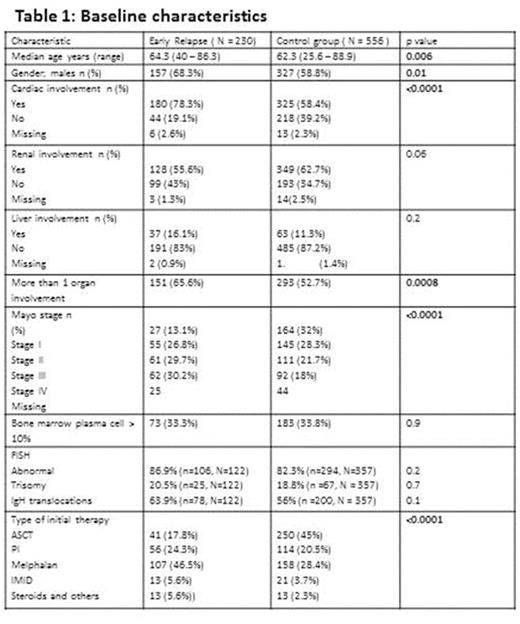
Seven hundred and eighty six patients with newly diagnosed systemic AL amyloidosis met the study criteria and were included in the analysis. Among these, 230 (29.3%) patients had ER within 12 months of starting initial therapy while 556 (70.7%) patients either relapsed after 1 year or had continuing response at the time of analysis. Baseline demographics, organ involvement and type of first line therapy are presented in Table1.
The median estimated follow up for the entire cohort from start of initial therapy was 62.9 months (95% CI; 59.9, 67.3). The variables included in the univariate and multivariate analyses for factors predicting ER were age at diagnosis (≤ vs > 70 years ), revised mayo stage (I and II vs III and IV), bone marrow plasma cell percentage (BMPC; ≤ 10% vs > 10%), presence of any chromosomal abnormalities, trisomies or IgH translocations by fluorescence in situ hybridization (FISH), multiorgan involvement [(>1 vs 1) (heart, liver, kidney, gastrointestinal tract, autonomic neuropathy), incorporation of ASCT in initial therapy.
In univariate analysis, mayo stage (p<0.0001), multiorgan involvement (p=0.0008) and inclusion of ASCT as part of initial therapy (p<0.0001) were significantly associated with ER, while age (p=0.06), BMPC(p=0.9), FISH abnormalities (p=0.2) were not. However, in multivariate analysis, only mayo stage (III + IV vs I + II; p=0.01) and non-inclusion of ASCT in first line treatment (p=0.0001) were significantly predictive of ER.
Conclusions
Despite the introduction of ASCT and novel drugs, the early mortality in systemic AL amyloidosis remains high. This study demonstrates that patients with ER are older with higher prevalence of cardiac involvement and multiorgan involvement and higher Mayo stage (III and IV). Incorporation of ASCT as part of the initial therapy was associated with reduced early relapse, but it is difficult to separate the influence of the eligibility for ASCT from the effect of ASCT itself. This will help us in characterizing these patients to better understand their mechanisms of resistance to therapy and gives an insight to the type of initial therapy that benefits them.
Dispenzieri:GSK: Membership on an entity's Board of Directors or advisory committees; Jannsen: Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Alnylam: Research Funding; pfizer: Research Funding. Kapoor:Takeda: Research Funding; Amgen: Research Funding; Celgene: Research Funding. Kumar:Celgene: Consultancy, Research Funding; Kesios: Consultancy; BMS: Consultancy; Sanofi: Consultancy, Research Funding; Noxxon Pharma: Consultancy, Research Funding; Glycomimetics: Consultancy; Millennium: Consultancy, Research Funding; Array BioPharma: Consultancy, Research Funding; Onyx: Consultancy, Research Funding; AbbVie: Research Funding; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.