Abstract
Cryopreserved HPC, CB products are maintained in long-term storage for future clinical use. To evaluate the impact of cryopreservation and storage over time on the quality and potency of the CBU as well as fulfill the FDA requirements for licensed products, stability studies are performed annually. These in vitro studies compare CBU stored for several years to recently processed ones with respect to HPCrecovery, potency, bacteriology, identity and integrity. This analysis summarizes the 2010-2015 annual stability evaluations including45 clinical CBU randomly selected among those processed by NCBP during the period 2006-2015 and stored inBioArchivefreezers.Pre-cryopreservation results, i.e., tests performed prior to freezing after CBU processing with the AXP system, are compared to post-thaw values of the CBU bag, and those of the CBU segment. We also present the quality assessment of segments (N=1924) of CBU processed during the same period that were released for transplantation.
Methods:CBU bags were thawed and underwent albumin dextran reconstitution (dilution 1:7).Total nucleated cell (TNC) counts were measured in aSysmexXE2100 analyzer. CD45+/CD34+ counts and viability were evaluated using single platform, 3-color flow cytometry with 7-AAD, and ISHAGE gating strategy.CFU assays were evaluated using the NCBP CFU strategy (Albano et al, ASH 2008).The same assays were used for segment evaluation. Recovery was expressed as the ratio of post-thaw to pre-cryopreservation values. The segment "yield" was calculated as the ratio of segment results to the pre-cryopreservation or post-thaw bag values.
Results: All CBU met acceptance criteria for identity, sterility and container integrity.
Post-thaw CBU bag TNC recovery averaged 100.5% (SD: 6.2%). The range was 87%-114.5%; only one sample had recovery below 90%. TNC was not measured routinely in the segments.
Average post-thaw bag CD34+ viability was 93.1% (SD: 3.2%). While the lowest value was 83.5%, 41/45 samples had viability above 90%. Segment post-thaw mean CD34+ viability was 94.7% (SD: 3.8%); 42 samples had values above 90%. The difference between segment and bag averaged 1.6% (SD: 2.5%; range: -3.1% to 7.5%). This change was statistically significant but too small to impact product quality.
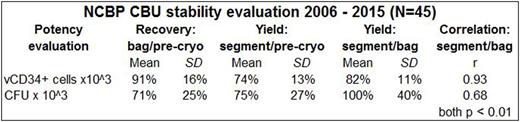
CD34+ and CFU recoveries (mean and SD) are shown in the Table. Post-thaw recovery of viable (v) CD34+ cells from theCBUbag ranged 60-133%, while the segment yield was 48-106%. For the 30 CBU with pre-cryopreservation CFU results, mean CBU bag recovery was 71% (range: 34%-120%) and segment CFU yield was 75% (range: 23%-125%). Segment and bag CD34+ and CFU results correlated well (p<0.01).
In agreement with the stability studies, CD34+ viability evaluation of 1924 CBU segments showed average of 95.7% (SD: 3.5%; only 5% of the samples had CD34+ viability below 90%). These segments were evaluated prior to CBU release for transplant; median time in the freezer was 2.3 years (range: 0.1-9.5), and they represent 4% of the total AXP-processed CBU in the NCBP inventory.
The segment yield of vCD34+ cells was 79% (SD: 25%). Further, a strong correlation was seen between pre-cryopreservation and segment vCD34+ counts (r: 0.87; p<0.01). CFU values of the segment and pre-cryopreservation also showed good correlation (r: 0.72; p<0.01) and the average CFU segment yield was 71% (SD: 24%).
CBU processed with manual method (period 1993-2006) are also included in annual stability studies and have met acceptance criteria (data not shown). Further, analysis of 684 segments of manually processed CBU stored for a median of 10 years (range: 6.3-21) showed mean CD34+ viability 94.2% (SD: 4.3%; 11% with viability below 90%); results that compare favorably to those of recently processed CBU.
In conclusion, systematic evaluation of NCBP CBU processed in different periods demonstrates that quality/potency can be maintained with storage over many years. The stability studies for the AXP-CBU (2006-to date) and the pre-release segment evaluation show high CD34+ viability and consistently high recovery of HPC, indicating that the process is under control, and set the standard for future studies and other potency assays.
The strong correlations between post-thaw bag and segment results demonstrate that the segment is a representative sample of the cryopreserved CBU and its evaluation can predict reliably the potency of the thawed product.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.