Abstract
Introduction: Melphalan based chemotherapy is commonly used for treatment of light chain amyloidosis (AL). Patients with AL often receive chemotherapy before autologous stem cell transplantation (ASCT) if they have high plasma cell burden or while awaiting ASCT. Melphalan is an alkylator and can affect bone marrow stem cells. Limited data is available on the effect of melphalan on stem cell mobilization in patients with amyloidosis. This study aims to identify the impact of melphalan therapy on collection of stem cells and ASCT in amyloidosis.
Methods: All patients with AL seen at our institution within 90 days of diagnosis over a 10-year period (2006 to 2015) who underwent stem cell harvest were identified from an institutional database. Data pertaining to demographics, diagnosis, treatment, stem cell harvest and ASCT was extracted from the electronic medical records. Analysis was carried out by chi-square and Fisher's exact test for categorical variables and Kruskal-Wallis and Wilcoxon rank sum test for ordinal and continuous variables.
Results: Three hundred and seventy two patients with AL who met the inclusion criteria were identified, of whom 10% (n=38) received melphalan based chemotherapy prior to harvesting, 28.5% (n=106) received non-melphalan based chemotherapy and 61.3% (n=228) received no chemotherapy prior to stem cell collection. Bortezomib based regimens were the most common (78%, n=83) non-melphalan based chemotherapy.
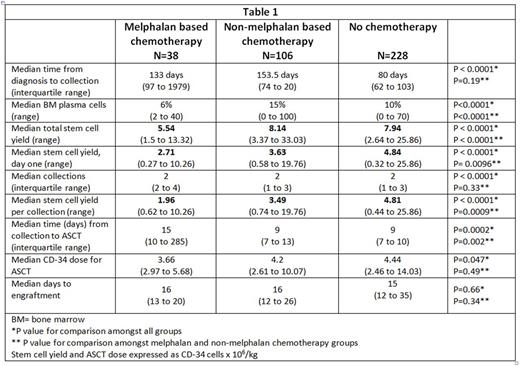
All three groups were similar in terms of median age at diagnosis (59.1 years), median age at collection (59.4 years), gender distribution (59% males, n=221) and type of involved free light chain (FLC), with lambda being more common (72.2%, n=268). Patients who received melphalan-based chemotherapy had more cardiac (73.8% vs. 45.2% vs. 46.4%, p=0.005) and renal (84.2% vs. 50.9% vs. 68%, p=0.0002) involvement compared to other chemotherapy and no chemotherapy groups, respectively. In contrast, patients who received non-melphalan based chemotherapy had higher plasma cell burden (15% vs. 6% vs. 10%, p< 0.0001) and greater difference between involved and uninvolved FLC (44.2 mg/dL vs. 13.3 mg/dL vs. 13.2 mg/dL, p< 0.0001) compared to melphalan and no chemotherapy, respectively.
Median duration of melphalan based chemotherapy was shorter at 54 days (34.5 to 79.5) or estimated 2 cycles compared to 101 days (60 to 135.5) or estimated 4 cycles (p=0.0019). Despite shorter duration of chemotherapy, total stem cell yield (million CD34/kg) was lower in patients who received melphalan based chemotherapy (5.54) compared to non-melphalan based chemotherapy (8.14) or no prior chemotherapy (7.94); p<0.0001. Similarly, day one stem cell yield (million CD34/kg) was the lowest in the melphalan group (2.71), followed by other chemotherapy group (3.63) and highest in no chemotherapy group (4.84); p<0.0001. This trend persisted for average stem cell yield per collection as illustrated in table 1.
Filgrastrim (GCSF) alone was the most common mobilizing agent. However, patients with any chemotherapy prior to harvesting had higher utilization of plerixafor; 26.3% (n=10) in the melphalan group and 39.6% (n=42) in the non-melphalan group compared to 11.6% (n=27) if no prior chemotherapy (p<0.0001). However, no statistically significant difference was seen for melphalan vs. non-melphalan chemotherapy groups (p=0.44).
In patients who underwent ASCT (85%, n=315), median stem cell dose (million CD34/kg) was different in the melphalan (3.66), non-melphalan (4.2) and no chemotherapy groups (4.44) (p=0.047), though the difference was not statistically significant amongst the 2 chemotherapy groups (p=0.34). There was also no difference in time to engraftment (table 1).
Conclusions: Melphalan based chemotherapy, even if used for a short duration of time, significantly decreases both total stem cell yield and the yield on day one. It therefore has the potential to add to resource utilization with more collections needed. As much as possible, limited cycles of melphalan based chemotherapy or non-melphalan based treatment should be utilized in patients who are transplant eligible.
Dispenzieri:GSK: Membership on an entity's Board of Directors or advisory committees; Alnylam: Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees; Jannsen: Research Funding; Celgene: Research Funding; pfizer: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kapoor:Amgen: Research Funding; Takeda: Research Funding; Celgene: Research Funding. Kumar:Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Research Funding; Glycomimetics: Consultancy; BMS: Consultancy; Sanofi: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Onyx: Consultancy, Research Funding; Array BioPharma: Consultancy, Research Funding; Noxxon Pharma: Consultancy, Research Funding; Kesios: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.