Abstract
Introduction
Hepatic veno-occlusive disease, also called sinusoidal obstruction syndrome (VOD/SOS), is a difficult to predict, and potentially life-threatening complication of conditioning for hematopoietic stem cell transplant (HSCT). VOD/SOS develops via a pathophysiologic cascade, and VOD/SOS with multi-organ dysfunction (MOD)/multi-organ failure (MOF) may be associated with >80% mortality. Defibrotide was recently approved in the United States for treating hepatic VOD/SOS with renal or pulmonary dysfunction post-HSCT and is approved in the European Union to treat severe hepatic VOD/SOS post-HSCT. In the United States, defibrotide had been available through an expanded-access program.
Methods
Patients in the expanded-access program were diagnosed with VOD/SOS by investigators using Baltimore criteria (bilirubin ≥2 mg/dL and ≥2 of the following: hepatomegaly, ascites, ≥5% weight gain), modified Seattle criteria (≥2 of the following: total bilirubin >2 mg/dL, hepatomegaly, or ascites and/or ≥5% weight gain [in this study]), or biopsy; elevated bilirubin was not required for patients with biopsy or who met modified Seattle criteria by presence of hepatomegaly with ascites/weight gain. This program included patients with or without MOD/MOF (defined by renal and/or pulmonary dysfunction). Defibrotide 25 mg/kg/day was given in 4 divided doses for a recommended ≥21 days. Here, Day +100 survival post-HSCT is explored post hoc based on 4 bilirubin-level categories at time of study entry; together these categories have been defined as 1 of the criteria in the proposed VOD/SOS grading scale for adults from the European Society for Blood and Marrow Transplantation (EBMT; ≥2 mg/dL to <3 mg/dL, ≥3 mg/dL to <5 mg/dL, ≥5 mg/dL to <8 mg/dL, and ≥8 mg/dL [Mohty M et al. Bone Marrow Transplant. 2016;51:906-912]). It is important to note that bilirubin <2 mg/dL is not part of the proposed EBMT criteria for VOD/SOS in adults.
Results
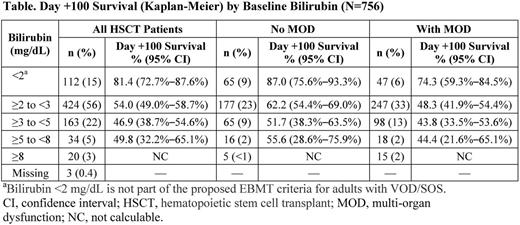
Among 756 post-HSCT patients enrolled through April 18, 2015, who received ≥1 dose of defibrotide, 427 also had MOD/MOF. Median age was lowest in patients with bilirubin <2 mg/dL (5 years). By comparison, median age in patients with bilirubin ≥2 to <3 mg/dL (56% of all patients) was 16 years; for bilirubin ≥3 to <5 mg/dL, median age was 13 years; and for the 2 small groups with bilirubin ≥5 to <8 or ≥8 mg/dL, median ages were 15 and 16.5 years, respectively.
Day +100 survival in the overall HSCT population of the expanded-access program was 55.4% by Kaplan-Meier estimate. The survival rate was 81.4% in patients with bilirubin <2 mg/dL; for patients with bilirubin levels ≥2 mg/dL, who tended to be older, survival estimates decreased (Table). Day +100 survival patterns by bilirubin level were generally similar in the subgroups of patients with and without MOD/MOF (Table).
Overall, 515 post-HSCT patients (67%) reported ≥1 adverse event (AE). Serious AEs were reported by 386 patients (50%), and AEs leading to death occurred in 250 patients (33%). Among all AEs, 158 patients (21%) had AEs that investigators assessed as related (possibly, probably, or definitely) to study medication.
Conclusions
Overall, higher bilirubin levels were associated with worse Day +100 outcomes (with the exception of the ≥5 to <8 mg/dL group, which represented only 5% of patients, and so has limited validity). However, interpretation of these results has to be treated with caution because only a single criterion from the EBMT guidelines was analyzed and because potentially confounding interactions (eg, age) were not assessed. Across all bilirubin-level categories, VOD/SOS with MOD/MOF was consistently associated with worse outcomes than VOD/SOS without MOD/MOF. These results further support the importance of identifying VOD/SOS earlier and suggest that diagnosis and treatment of VOD/SOS, before bilirubin becomes markedly elevated, may be associated with improved outcomes.
Support: Jazz Pharmaceuticals.
Richardson:Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kernan:Gentium: Research Funding; The National Cancer Institute of the National Institutes of Health: Research Funding. Grupp:Novartis: Consultancy, Research Funding; Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy. Antin:Gentium SpA/Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Liang:Jazz Pharmaceuticals, Inc.: Employment, Other: stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Hume:Jazz Pharmaceuticals, Inc.: Employment, Other: stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Tappe:Jazz Pharmaceuticals, Inc.: Employment, Other: stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc. Soiffer:GentiumSpA/Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.