Abstract
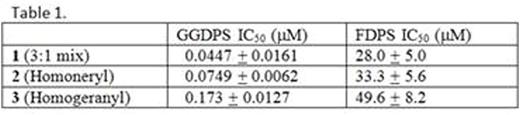
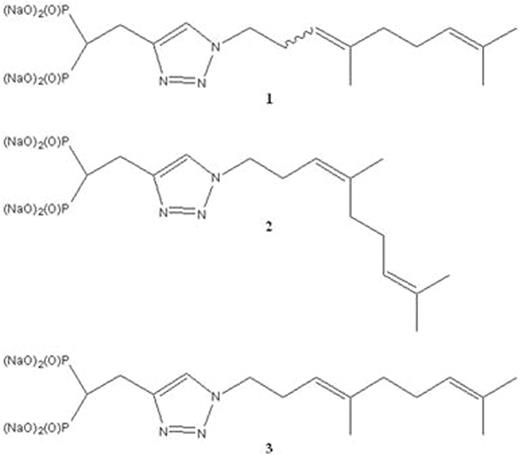
The production of monoclonal protein (MP) by malignant plasma cells is a hallmark of multiple myeloma (MM). We have previously demonstrated that targeting the Rab GTPase family via disruption of the post-translational modification geranylgeranylation impairs intracellular monoclonal protein trafficking, leading to endoplasmic reticulum stress and apoptosis. Thus, inhibition of Rab geranylgeranylation represents a novel therapeutic strategy in MM. One approach by which to disrupt protein geranylgeranylation is through inhibition of the enzyme geranylgeranyl diphosphate synthase (GGDPS), which catalyzes the synthesis of the 20-carbon geranylgeranyl diphosphate (GGDP) from the 15-carbon farnesyl diphosphate (FDP) and the 5-carbon isopentenyl diphosphate (IPP). We have previously reported a series of triazole bisphosphonates and their activity as GGDPS inhibitors. The lead compound in this series was found to be the triazole 1 which was initially prepared as a mixture of isomers in a 3:1 ratio of the homogeranyl:homoneryl isomers and was found to be the most potent GGDPS inhibitor published to date with an IC50 of 45 nM. Our prior work involving geranyl and neryl triazoles had revealed that the neryl isomer was significantly more potent than the geranyl and that the mix of those two isomers had intermediate activity. We therefore hypothesized that the homoneryl isomer 2 would prove to be more potent than the homogeranyl isomer 3. To this end, each individual isomer was prepared in pure form and subjected to in vitro enzyme assays for GGDPS and the related enzyme FDPS. Cellular activity in human myeloma cell lines (RPMI-8226, MM.1S) was assessed via immunoblot analysis of unmodified Rap1a (as a representative geranylgeranylated protein) as well as ELISA of intracellular levels of lambda light chain (as a marker for disruption of Rab geranylgeranylation). As shown in Table 1, while 2 was found to be a more potent inhibitor of GGDPS than 3, interestingly, it was less potent than the original 3:1 mixture (1). The isomers only weakly inhibited FDPS, revealing specificity for GGDPS. Immunoblot analysis and ELISA studies also demonstrated that 2 more potently disrupts cellular geranylgeranylation than its olefin isomer 3. In addition, measurement of intracellular GGPP levels confirmed that 2 more potently decreased cellular GGPP levels than 3. To investigate why the individual isomers were less potent than the original mixture, studies were performed in which cells were treated with varying concentrations of 2 and 3 both alone and in different combinations. These studies revealed a synergistic increase in both unmodified Rap1a and intracellular light chains when the cells were treated with a 3:1 mixture of 3 and 2, respectively. However, no such increase was observed with either a 1:3 mixture or a 1:1 mixture. Notably, this synergy between the two isomers was also observed in the GGDPS enzyme assay (combination index for EC50 of 0.25), suggesting that the two isomers might bind simultaneously to the active site but within different domains. Using computer modeling and available crystallography data, it was revealed that 2 is preferred at the FPP site and binds with very high affinity while 3 demonstrates a preference for the GGPP site and that both may bind to the enzyme simultaneously. Crystallographic studies involving the protein and the isomer mixture are now underway to confirm the results of the modeling studies. The activity of 2 and 3, both alone and in combination could be further potentiated by co-incubation with submicromolar concentrations of lovastatin, an HMG-CoA reductase inhibitor. This has clinical significance as submicromolar levels of statins are readily achieved with standard therapeutic dosing and thus could be used to enhance activity of the novel drugs in vivo. In conclusion, these results are highly intriguing as they demonstrate olefin stereoisomers that have synergistic inhibitory activity against the target enzyme GGDPS. These studies establish homogeranyl/homoneryl triazole bisphosphonate 1 as the lead GGDPS inhibitor as a potential therapeutic agent for multiple myeloma and provide new directions for future development of inhibitors which are designed to simultaneously interact with multiple binding sites within the target enzyme.
Wiemer:Terpenoid Therapeutics, Inc.: Other: Founder. Holstein:Celgene: Membership on an entity's Board of Directors or advisory committees; Millennium: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.