Abstract
Introduction: PREAMBLE (Prospective REsearch Assessment in multiple Myeloma: an oBservationaL Evaluation) is an ongoing, prospective, multinational, non-interventional observational study to collect information from medical records regarding treatment and diagnostic procedures. By analyzing these data, a better understanding can be gained of the real-world effectiveness of novel agents used in treating relapsed/refractory multiple myeloma (RRMM) and their impact on patient-reported outcomes. This analysis was performed to evaluate comorbidities, treatment patterns, and survival in a PREAMBLE subpopulation that included US patients with MM.
Methods: US patients aged ≥18 years with RRMM, ≥1 prior therapy, and initiating treatment with an immunomodulatory drug (IMiD), proteasome inhibitor (PI), or IMiD+PI within 90 days before or 30 days after study enrollment (index therapy) were identified from PREAMBLE. Data were collected at the index healthcare provider visit and every 3 (Year 1) and 6 (Years 2-3) months (mo) over 3 years or until the end of patient follow-up. Data were summarized using descriptive statistics and included age, sex, International Staging System (ISS) stage, type of visits and hospitalizations, disease status, line of therapy, and concomitant disorders. Overall survival (OS) and progression-free survival (PFS) were analyzed using Cox regression and Kaplan-Meier techniques. MM-specific survival was calculated using Cumulative Incidence Function.
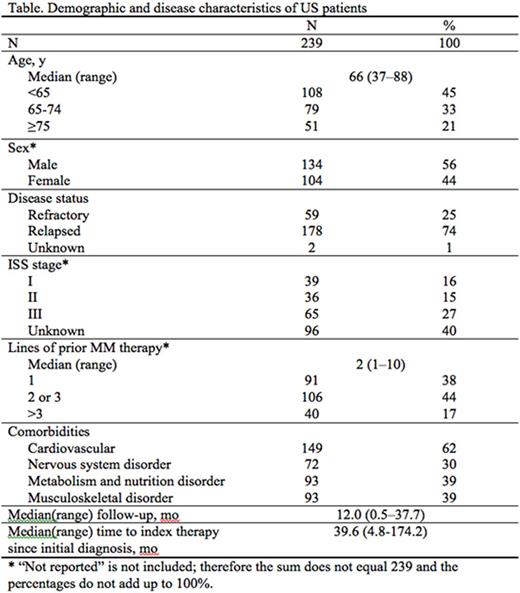
Results: 239 patients (median age 66 years; 56% male) were enrolled. At the time of data cut-off (7 December 2015), 124 (52%) were still in the study and 115 (48%) had disenrolled, including 71 (62%) who had disenrolled due to death. Median follow-up was 12.0 mo (range, 0.5-37.7). At study entry, 178 (74%) patients had relapsed MM; 140 had ISS stage assigned, with 28% (n=39), 26% (n=36), and 46% (n=65) for Stage I, II, and III disease, respectively. Median time to index therapy since initial diagnosis was 39.6 mo (range, 4.8-174.2). Cardiovascular disease (CVD) was the most common comorbidity (62%) and other comorbidities were highly prevalent as well (Table).
At enrollment, most patients were receiving a PI (n=136, 57%; carfilzomib n=68/136, 50%) or an IMiD (n=63, 26%; pomalidomide n=29/63, 46%); 40 patients (17%) were receiving an IMiD+PI (carfilzomib and/or pomalidomide n=11/40, 28%). Median number of prior therapies was 2 (range, 1-10).
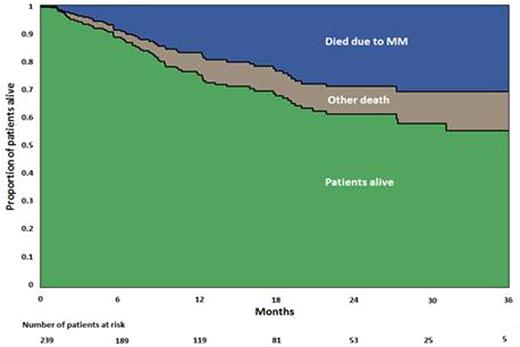
The overall median PFS was 7.9 mo, with a median PFS for second-line, third-line, and fourth- or greater line of 15.4, 9.0, and 6.9 mo, respectively. Median OS has not yet been reached. 18-mo all-cause mortality was 31.1% (95% CI 24.9-38.5), while 18-mo MM-related mortality was 22.3% (95% CI 16.2-28.4), meaning that at 18 mo, approximately 8.8% of patients died due to non-MM-associated causes (Figure). Death rates were independent of comorbidities and number of lines of prior therapy. A strong association was found between disease status and rate of death: refractory disease was associated with higher death rates (hazard ratio 1.92; 95% CI 1.16-3.16), while sex and ISS stage showed weak association with death rates.
Conclusions: The progressively shorter PFS with each successive treatment regimen reflects ongoing development of drug resistance. In this preliminary survival analysis of US patients, the majority of deaths resulted from MM. This finding demonstrates the need for additional treatment options for treating RRMM. CVD was highly prevalent as a comorbidity across all lines of treatment.
Vij:Jazz: Consultancy; Karyopharma: Consultancy; Shire: Consultancy; Takeda: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Celgene: Consultancy; Bristol-Myers Squibb: Consultancy; Janssen: Consultancy; Novartis: Consultancy. Popov:PAREXEL: Employment. Chen:Bristol-Myers Squibb: Employment.
Author notes
Asterisk with author names denotes non-ASH members.