Abstract
Introduction
Immunomodulatory drugs (IMiDs) such as the newer-generation agents lenalidomide and pomalidomide have become essential treatment backbones for myeloma, but some patients may experience skin and other toxicity resulting in discontinuation of these life-prolonging drugs. The optimal management of moderately severe cutaneous and other potentially life-threatening allergic reactions (including angioedema and anaphylaxis), has not been defined. Beginning in 2011, we developed a rapid desensitization program (RDP) for patients who developed significant cutaneous/allergic reactions (Grade 3 CTCAE 4.03 hypersensitivity reactions [HSRs]) felt to contraindicate re-exposure to an IMiD by the treating physician. Patients who had Grade 2 or less cutaneous HSRs were not eligible for RDP. Our primary objective of the RDP was to safely restore treatment with these key agents.
Methodology
Patients with only mild or grade 1/2 (CTCAE 4.03) HSRs to prior IMiDs were excluded. After obtaining informed consent, patients who had experienced more severe reactions were admitted to a step-down nursing facility with an anaphylaxis kit at bedside. As per protocol, a 10-14 step RDP process took on average 3.5-5 hours to complete (Seki J, et al. ClinLymphoma Myeloma Leuk 2013; 13: 162-5 and Seki J, et al. J Clin Med Res 2015; 7:807-811 2015). Capsules of the target IMiDs were emptied and dissolved into an Erlenmeyer flask or beaker containing either saline solution (in the case of lenalidomide), or an institution-developed suspension (for pomalidomide), from which 3 or 4 different concentrations of stock solutions were prepared; the desired strengths produced were dependent on the target dose for a given patient. The RDP protocol also contained monitoring guidelines for frequent vital signs and rescue medications in case of breakthrough reactions. Post-RDP, patients were observed and monitored overnight. If no problems were encountered, patients were re-challenged with the IMiD full target dose the next morning. Patients were discharged if no active issues developed in the following 4 hours. Longitudinal follow-up by phone at one-, two- and four weeks post-discharge continued to ensure safety and efficacy of RDP. Post-assessment, patients were instructed to report worsening of adverse reactions to the myeloma triage nurse by phone and/or seek urgent care when needed.
Results
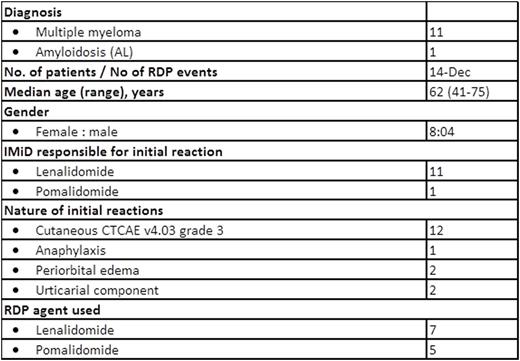
No one developed irreversible or serious adverse reactions during the procedures. One (1/12) patient developed a recurrent mild rash around her forehead and temporal area which migrated to the back of her neck a few hours post-RDP which lasted into the next day. The rash waxed and waned but resolved at the end of the day without intervention. Two (2/12) patients had systolic and diastolic blood pressure (BP) fluctuations during the procedure, especially at the early stages of the desensitization. BPs normalized in both patients before completing the RDP. Patients tolerated the contents of the oral syringes containing the two different IMiDs formulation well. A second RDP was offered to two patients. One, who had developed milder forms of HSR following the first procedure compared to the initial reaction (but still at least Grade 2), underwent a second RDP but developed recurrent hives 3 weeks post-discharge that could not be controlled with antihistamines; the second had developed angioedema and rash after the first RDP and was premedicated with prednisone, antihistamines and montelukast before the second. Subsequently, this patient was able to continue lenalidomide until disease progression. No other patients were premedicated prior to commencing RDP.
Conclusion
Our RDP was a safe and effective means to manage IMiD-related HSRs that were moderately severe in nature. In total our RDP protocol allowed eleven of twelve patients (92%) with significant HSRs, felt to preclude further IMiD treatment, to proceed with such therapy without IMiD-related toxicity. Given the critical need for IMiD treatment in myeloma and amyloidosis, we feel this program improved patients' outcomes with minimum risk.
Patient characteristics
Results of RDP
Trudel:Celgene: Honoraria; Glaxo Smith Kline: Honoraria, Research Funding; Novartis: Honoraria; Oncoethix: Research Funding. Reece:Otsuka: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.