Abstract
Introduction:
The global distribution of sickle cell disease (SCD) overlaps with that of food scarcity and nutritional deficiencies. Chronic anemia secondary to SCD contributes to nutritional deficiencies, for example folic acid. Few studies have evaluated associations between malnutrition and SCD, nor effects of malnutrition on SCD outcomes. Moreover,hydroxyurea(HU), a disease modifying agent for SCD, has not been specifically evaluated in malnourished children although it is known to bind pharmacologically to human serum albumin.
Methods:
We conducted a cross-sectional nutritional assessment of children receiving chronic SCD care at a referral hospital in the capital city of Malawi, Lilongwe, between January and June 2016. Participants were measured for height, weight, and mid-upper arm circumference. Z-scores and percentiles for anthropomorphic indices were calculated using WHOAnthroSoftware. Nutritional measures of interest were BMI-for-age Z-score to assess wasting and height-for-age Z-score to assess stunting. We examined associations between nutritional status and clinical and laboratory data collected longitudinally at six-month intervals during routine care. Differences based on HU use were examined.
Results:
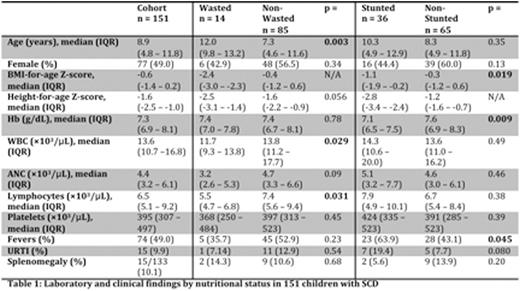
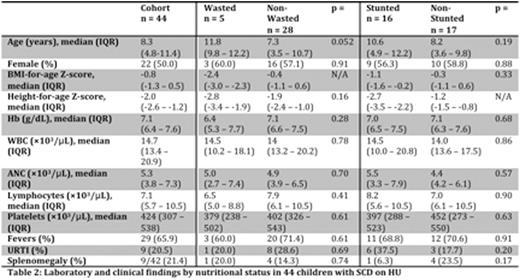
Fourteen of 99 (14.1%) participants had moderate-to-severe wasting and 36 of 101 (35.6%) moderate-to-severe stunting. The wasted group was significantly older than the non-wasted group but there was no significant difference in age between the stunted and non-stunted groups (Table 1). We observed significantly lower white blood cell (WBC) and lymphocyte counts in the wasted group when compared to the non-wasted group. The stunted group had significantly lower hemoglobin (Hb) than the non-stunted group. There was also a significantly higher prevalence of fevers in the stunted group than the non-stunted group. Forty-four of 151 (29.1%) participants were receiving HU for a median duration of 126 days (IQR 29 - 196 days). There were no significant differences in age or sex between the HU group and the non-HU group. We observed significantly lower Hb in the HU group when compared to the non-HU group, as expected as HU is typically prescribed for children with more severe SCD in Lilongwe. We also observed higher WBC, absolute neutrophil (ANC), and lymphocyte counts in the HU group. Again reflecting greater SCD severity, we observed a significantly more frequent history of fevers, upper respiratory tract infections, and splenomegaly in the HU group. There were no significant differences in the proportions of wasted participants by HU status [5/33 (15.2%) HUvs20/68 (29.4%) non-HU, p=0.84], and a trend toward more frequent stunting among HU users again likely reflecting growth effects of greater chronic SCD severity [16/33 (48.5%) HUvs9/66 (13.6%) non-HU, p=0.061]. Among HU users, peripheral blood counts were not significantly different in wastedvsnon-wasted children, nor stuntedvsnon-stunted children (Table 2).
Conclusion:
In a pediatric SCD cohort in Malawi we observed 14.1% of children with significant wasting and 35.6% with significant stunting, highlighting growth effects of chronic illness in this population even among children receiving regular SCD care. Among children prescribed HU, nutritional status did not seem to be associated with HU tolerability. Notably, HU was not associated with significantmyelosuppressionin our cohort including those children with wasting or stunting. More studies are needed to clarify interactions between nutritional status, SCD, and HU, but our data suggests wasting or stunting should not be contraindications to HU use when indicated for pediatric SCD in sub-Saharan Africa.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.