Abstract
Background
Invasive fungal infections (IFI) are serious and life-threatening complications of hematological malignancies particularly in patients affected by acute myeloid leukemia and following hematopoietic stem cell transplantation. However, only few epidemiological data concerning fungal infections in chroniclymphoproliferativedisorders are available.
In this work we aimed to identify the clinical and biological features of patients with chronic lymphocytic leukemia (CLL) affected by IFI.
Methods
We performed a single-center retrospective study based on the CLL patients referred to the Hematology Unit of University of Padua from 1983 to 2015. IFI were defined as infective events caused by yeasts ormouldsthat required inpatient management and/or intravenous antifungal therapies. We included only proven and probable IFI. Time to IFI (TIFI) and overall survival (OS) were calculated from the date of CLL diagnosis to IFI development or death (event), respectively, or last available follow-up (censored). Diagnostic work-up of fungal infection included blood cultures, serumgalactomannanantigen test for 3 consecutive days (cut-off 0.5), β-1,3-(D)-glucanand high resolution chest computed tomography andbronchoalveolarlavages when clinically indicated.
We also collected the closest immunoglobulin (IG) levels data before the onset of each IFI or serious bacterial infective events. IGs measured during infections were not included in the analysis, because they could have been influenced by the infectious event. The last available serum IG levels were considered for patients who did not suffer from any IFI.
Results
795 patients with CLL were recruited in this study. 60% were male and the median age at diagnosis and at IFI onset were 65 and 68 years, respectively. The median follow-up was 8.5 years and 316 patients required treatment during the follow-up.
11 out of 795 (1.4%) patients developed IFI during the follow-up. Among them, 7 patients (64%) had probable Aspergillosisand 4 had proven IFI (3 Aspergillusspp and 1 Candida kruseii) based on histological confirmation and/or microbiological isolation of the specific fungal agent. 9 patients wereBinet stage A, 2Binet B and noneBinet C at CLL diagnosis. The median lines of treatment were 5 ranging from 2 to 9. Six out of 11 (55%) patients showed high-risk cytogenetic by FISH analysis (11q or 17p deletion), 2 harbored complex karyotypes, 62% (5/8) hadunmutated IGVH gene.
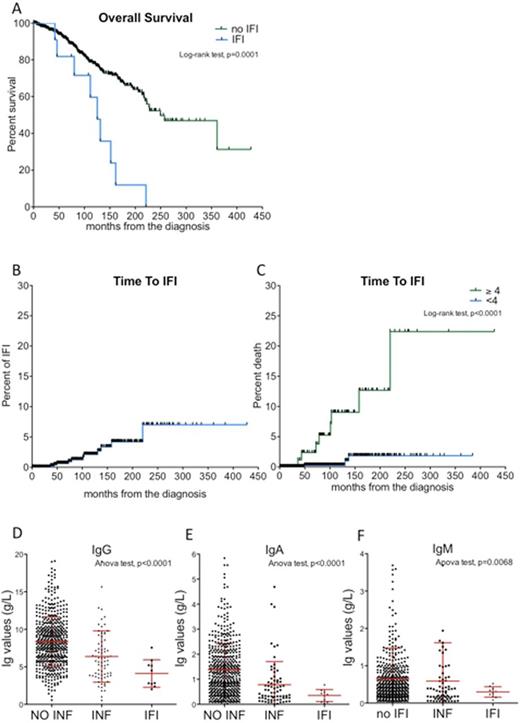
By Kaplan-Meyer analysis we showed that IFI occurred in 2.1% and 7.0% of the cohort after 10 and 20 years of follow-up, respectively (Figure 1A). However, considering the number of chemotherapy regimens, the estimated cumulative incidence of IFI was higher in patients who were treated with at least 4 lines of chemo-immunotherapy (10-year TIFI were 9.0% and 0.3%, p<0.0001, Figure 1B).
IFI occurrence was associated with a very poor prognosis. In fact, the median overall survival of patients who developed IFI was 125 months as compared to 250 months of the remaining cohort (Log-rank test, p=0.0001, Figure 1C). Since IFI was itself the main cause of death of these subjects. In multivariable analysis, patients with IFI had almost 10 times higher-risk of death (HR 10, p=0.0012) that other patients.
We demonstrated that, at the time of IFI events, patients had lower levels of IG as compared to a group of 72 patients who had serious bacterial infections or to 712 subjects who did not have any infections. The medianIgGlevels were 4.11, 6.38 and 8.38 g/L for patients with IFI, bacterial infections and without history of infections, respectively (ANOVA test, p<0.0001, Figure 1D). The median IgA levels were 0.35, 0.78 and 1.39 g/L for patients with IFI, bacterial and none infections, respectively (ANOVA test, p<0.0001, Figure 1E). The medianIgMlevels were 0.28, 0.59 and 0.65 g/L for patients with IFI, bacterial and none infections, respectively (ANOVA test, p=0.0038, Figure 1F).
Conclusions
Herein, we described the epidemiology of invasive fungal infections among a large cohort of CLL patients from a single institution over 32 years. We demonstrated that IFI significantly impacts on the survival of CLL patients and we provided evidence of the importance of previous chemo-immunotherapy and IG levels on the risk of developing IFI.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.