Abstract
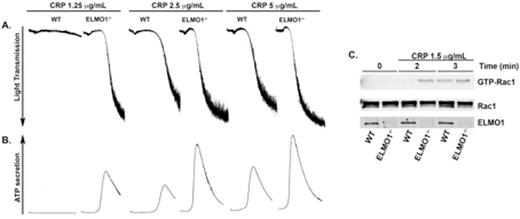
PI3-kinase (phosphoinositide 3-kinase) is an important signaling molecule that is activated downstream of various receptors upon platelet activation. PI3-kinase activation leads to the generation of PIP3 (Phosphatidylinositol (3,4,5)-trisphosphate) subsequently leading to the recruitment of PH (pleckstrin homology) domain containing proteins to the plasma membrane. Our laboratory screened for proteins that interacted with PIP3 (Phosphatidylinositol (3,4,5)-trisphosphate) using PIP3 beads. One of the proteins that interacted with PIP3 was ELMO1 (Engulfment and cell motility-1). ELMO1 is a scaffold protein with no catalytic activity and is well known to regulate actin cytoskeletal rearrangement via Rac1 in other cells. However, it is not known whether ELMO1 is expressed in platelets and if so, does it regulate platelet functional responses. Here, we show that ELMO1 is present in both human and murine platelets. We used ELMO1-deficient (ELMO1-/-) mice to study its role in platelets. ELMO1-/- murine platelets showed enhanced platelet aggregation and dense granule secretion in response to the GPVI agonist, CRP (Figure 1 A & B), compared to the wildtype controls although there was no difference in GPVI expression levels between the two. There was no difference observed in response to AYPGKF- or 2-MeSADP. These data suggest that ELMO1 plays a specific role downstream of GPVI pathway but GPCRs. Moreover, ELMO1-/- platelets exhibited enhanced clot retraction and spreading indicating its role in Glycoprotein IIb/IIa (GPIIb/IIIa) mediated outside-in signaling. Furthermore, whole blood from ELMO1-/- mice perfused over collagen under arterial shear conditions exhibited enhanced thrombus formation. In an in vivo pulmonary thromboembolism model, ELMO1-/- mice showed reduced survival compared to the wildtype control. ELMO1-/- mice also showed shorter time to occlusion and increased thrombus stability using the ferric-chloride injury model indicating the role of ELMO1 in thrombus formation in vivo. At the molecular level, Rac1 activity was enhanced in ELMO1-/- murine platelets compared to the wildtype control in response to CRP (Figure 1C). Together, these data suggest that ELMO1 regulates Rac1 activity upon GPVI-mediated thrombus formation and it may play a negative regulator role in both inside-out and outside-in signaling, which might involve Rac1.
Representative figure of (A) platelet aggregation and (B) dense granule secretion. (C) Washed platelets were stimulated with CRP 1.25 μg/mL for the indicated times. GST-PAK-RBD was used to pull-down active Rac1 from platelet lysates and was detected using specific antibody to Rac1 by Western blot. WT = Wildtype mice. ELMO1-/- = ELMO1-deficient mice. CRP = collagen related protein.
Representative figure of (A) platelet aggregation and (B) dense granule secretion. (C) Washed platelets were stimulated with CRP 1.25 μg/mL for the indicated times. GST-PAK-RBD was used to pull-down active Rac1 from platelet lysates and was detected using specific antibody to Rac1 by Western blot. WT = Wildtype mice. ELMO1-/- = ELMO1-deficient mice. CRP = collagen related protein.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.