Abstract
The CLEC-2 agonist, rhodocytin, elicits powerful platelet activation signals in conjunction with Src family kinases, Syk, and PLCg2. Previous reports have shown that rhodocytin-induced aggregation is dependent on secondary mediators. However, the role of secondary mediators in CLEC-2 signaling is not defined. In this study we report that CLEC-2-induced Syk activation is aspirin-sensitive and that TxA2 plays an important role in the most proximal events such as CLEC-2 phosphorylation and Syk activation. We also show that the activation of other GPCRs, such as the ADP receptors and PARs, can also potentiate CLEC-2 signaling. By using the Gq-inhibitor, UBO-QIC, or P2Y1/P2Y12 antagonists, we show that the Gq-signaling, but not G12/13 or Gi, is essential for GPCR-induced Syk phosphorylation downstream of CLEC-2. We further elucidated the signaling involved in Gq-mediated Syk phosphorylation and identified an important role for PKCs downstream of PLCb regulating SFK activation (Figure 1A). Using Lyn-knock out murine platelets we identified a potential role for Lyn downstream of the Gq-pathway in potentiating CLEC-2 signaling by using. We suggest that, at low concentration of CLEC-2 agonist, the unclustered CLEC-2 receptors are phosphorylated by the Gq-activated Lyn resulting in the potentiation of the initial CLEC-2 signal (Figure 1B). However, Gq receptors by themselves cannot phosphorylate CLEC-2 receptors and require minimal levels of ITAM-containing receptor stimulation in order to initiate unclustered CLEC-2 receptor phosphorylation. Together, these results provide evidence for a novel Lyn-mediated regulation of CLEC-2 signaling by Gq-coupled receptors thereby leading to potentiation of CLEC-2-induced signaling (Figure 1C).
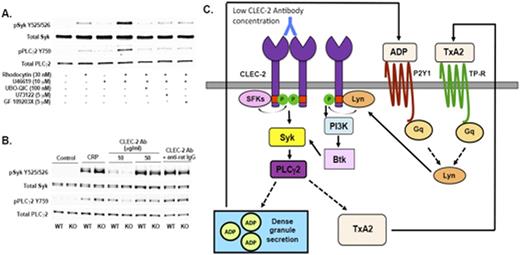
A) Western blot showingeffect Gq-pathway inhibitors on CLEC-2 signaling. B) Effect of low concentration of CLEC-2 agonist in Lyn-knock out murine platelets. C) Model showing role of Gq-activated Lyn in CLEC-2 signaling.
A) Western blot showingeffect Gq-pathway inhibitors on CLEC-2 signaling. B) Effect of low concentration of CLEC-2 agonist in Lyn-knock out murine platelets. C) Model showing role of Gq-activated Lyn in CLEC-2 signaling.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.