Abstract
Multiple myeloma (MM) is an incurable plasma cell disorder representing 10% of all hematologic malignancies. Cancer is a known risk factor for venous thromboembolism (VTE). Patients with MM are at a particularly high risk of developing VTE owing to patient characteristics (e.g. previous history of VTE), disease characteristics, and treatment characteristics including use of the immunomodulatory agents (IMIDs). Unfortunately, standard criteria to identify the patients most at risk for developing VTE in MM while receiving IMIDs are unknown. We sought to assess the incidence of VTE and its associated risk factors in MM patients receiving IMID therapy.
A retrospective cohort study including 1680 consecutive patients with multiple myeloma treated at our centre between January 01, 1995 and January 26, 2016 was conducted. The annual incidence of VTE on immunomodulatory agents including thalidomide, lenalidomide, and pomalidomide was derived. Univariate incidence ratio analyses of VTE for different risk factors was performed including: previous history of VTE, concomitant use of dexamethasone, and ≥/< 6 months after IMID initiation.
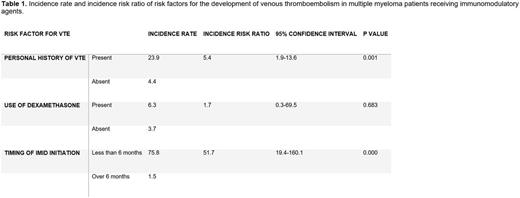
A total of 309 MM patients treated with an immunomodulatory agent were identified. Nineteen patients were excluded (incomplete data, lost to follow). Of the remaining 290 patients, the mean age was 67.9 and 42.4% were female. Twenty-seven VTE events were recorded. The overall risk ratio was 6.1 for the development of VTE. Patients with a personal history of VTE had an increased risk of suffering a VTE while on IMID therapy (IRR 5.4; CI, 1.9-13.6). The time from the initiation of the IMID therapy (less than 6 months) also increased the risk of developing a VTE (IRR 51.7; CI,19.4-160.1). The concomitant use of dexamethasone was not associated with a statistically significant increased risk (IRR 1.7; CI, 0.3-69.5). Incidence risk ratios for these risk factors are depicted in Table 1.
Our results suggest that a personal history of VTE and the time from the initiation of the IMID (less than 6 months) are associated with an increased risk of VTE in MM patients receiving IMID therapy. This may be helpful in determining which multiple myeloma patients treated with an IMID agent warrant more aggressive thromboprophylaxis. Further prospective studies are needed to determine the optimal agent, intensity, and duration of thromboprophylaxis in patients with MM on IMID therapy.
McCurdy:Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.