Abstract
Acute myeloid leukemia (AML) is an aggressive hematologic neoplasm characterized by clonal expansion of myeloid blasts. The FMS-like tyrosine kinase-3 (FLT3) receptor gene is the most commonly mutated gene in AML, and patients who harbor a FLT3/ITD mutation have a relatively poor prognosis. FLT3/ITD activates Rac1 GTPase, and both FLT3/ITD and Rac1 play key roles in the signaling network that activates signal transducer and activator of transcription 5 (STAT5) and promotes leukemogenesis.
Utilizing a co-immunoprecipitation/mass spectrometry analysis, we found that FLT3/ITD interacts with Dedicator of Cytokinesis 2 (DOCK2), which is a guanine nucleotide exchange factor for Rac GTPases. Expression of DOCK2 is limited to hematopoietic cells, and it is expressed in leukemic blasts of patients with FLT3/ITD AML. Knockdown (KD) of DOCK2 results in reduced Rac1 activity and leads to decreased survival of leukemic cells with elevated FLT3 activity, both alone and in combination with cytarabine (Ara-C) treatment in both in vitro studies and a mouse xenograft model.
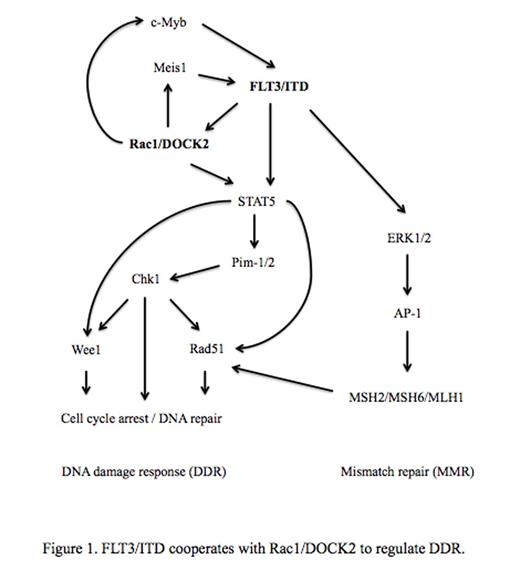
We further investigated the mechanisms by which Rac1/DOCK2 activity affects cell survival and chemotherapeutic response in FLT3/ITD leukemic cells. Exogenous expression of FLT3/ITD in TF-1 cells resulted in elevated Rac1 activity, an increase in the level of reactive oxygen species, and increased activation of STAT5. Western and qPCR studies in these cells demonstrated a concomitant statistically significant increase in DNA damage response (DDR) factors, including Chk1, Rad51, Wee1, and PIM-1. Furthermore, expression of FLT3/ITD led to increased expression of AP-1 and its downstream targets, the mismatch repair (MMR) factors MSH2, MLH1 and MSH6. These FLT3/ITD-expressing TF-1 cells exhibited markedly increased resistance to Ara-C treatment, and the increase in IC50 positively correlates with the expression level of FLT3/ITD. These effects on DDR factors appear to be dependent on FLT3/ITD activity, as they were not observed in TF-1 cells expressing even relatively high levels of wild-type (WT) FLT3. These findings suggest that enhanced DDR may provide a mechanism of resistance to Ara-C treatment in FLT3/ITD cells.
We then utilized DOCK2 KD in patient-derived leukemia cell lines that express FLT3/ITD (MV4;11 and Molm 14) to evaluate whether decreased Rac1 activity would restore sensitivity to Ara-C. DOCK2 KD via shRNA resulted in significantly reduced expression of Meis1 and c-Myb, which are known regulators of FLT3 expression. Accordingly, the expression level and activity of FLT3 in cells with DOCK2 KD are markedly decreased. The DOCK2 KD cells showed reduced STAT5 activity and decreased expression of Chk1, Rad51, Wee1 and PIM-1, as well as lower expression levels of AP1, MSH2, MSH6 and MLH1. Consistent with the reduction in activity of DDR factors, DOCK2 KD cells exhibited significantly increased sensitivity to Chk1 inhibitor MK8776, Wee1 inhibitor MK1775 and Rad51 inhibitor B02. Moreover, synergistic effects between these DDR inhibitors and Ara-C, as indicated by increased apoptosis and reduction in cell proliferation, were observed at markedly lower concentrations in FLT3/ITD cells with DOCK2 KD. In contrast, DOCK2 KD in a leukemia cell line expressing WT FLT3 (REH) did not lead to down-regulation of the activity of FLT3 or DDR factors, and no enhancement in sensitivity toward DDR inhibitors was observed.
These findings suggest that FLT3/ITD and Rac1 activity cooperatively modulate DDR and MMR activity in leukemic cells. The increased DNA damage repair activity of FLT3/ITD leukemia cells may play a role in the relatively poor response of FLT3/ITD AML to standard AML chemotherapeutic regimens. Therefore, the addition of DDR inhibitors to conventional chemotherapy may be useful in the treatment of FLT3/ITD AML, and pharmacologic inhibition of the Rac signaling pathways via DOCK2 may provide a novel and promising therapeutic target for FLT3/ITD AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.