Abstract
Background
The percentage of blasts in the bone marrow in the early stage of remission induction therapy for acute myeloid leukemia (AML) has been reported to affect not only remission rate, but also long-term prognosis. If early post-treatment response to therapy was determined to affect prognosis, this finding could be a basis for selection of a proactive treatment such as hematopoietic stem cell transplantation; however, the proper assessment period and the percentage of blast cell survival vary among reports. Therefore, we retrospectively analyzed the relationship between prognosis and the percentage of blasts in the bone marrow in the early post-treatment stage in cases registered for the AML201 study conducted by the Japan Adult Leukemia Study Group (JALSG).
Patients and Methods
The JALSG AML201 study was a multicenter randomized study in which 1064 patients with newly diagnosed AML patients were registered between December 2001 and December 2005. For remission induction therapy, we compared standard-dose idarubicin or high-dose daunorubicin in combination with cytarabine. For consolidation therapy, we compared standard-dose multiagent chemotherapy or high-dose cytarabine alone, and no apparent differences were observed in overall survival (OS) between both treatment groups. The percentage of blasts in the bone marrow on day 15 was strongly associated with remission; using the receiver operating characteristic curve as a reference, we divided patients into a ≥5% and a <5% blasts group and compared their prognoses. For between-groups comparisons, we used Fisher's exact test. For comparison of survival curves, we employed the log-rank test. We conducted statistical analysis using the statistics program EZR.
Results
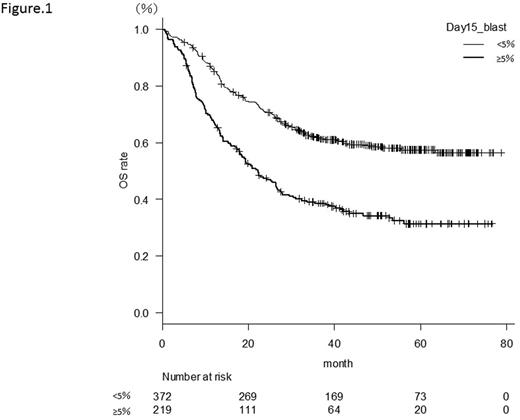
Among the 1064 patients registered for the JALSG AML201 study, 600 patients for whom bone marrow examination was performed on Day 15 were selected as subjects for the present study. The patients consisted of 375 men and 225 women with a median age of 47 years (15-64 years). A total of 377 patients (62.8%) had <5% blasts in their bone marrow on day 15 and their complete remission (CR) rate was 91.8%. On the other hand, 223 patients (37.2%) had ≥5% blasts and their CR rate was 58.3%. The 3-year overall survival rate (OS) in the <5% blasts group and the ≥5% blasts groups were 61.6% and 38.6%, respectively (p < 0.001) (Figure 1); while the 3-year relapse free survival rate (RFS) was 46.3% vs. 29.8%, respectively (p < 0.001). Thus, the group with <5% blasts in the bone marrow showed significantly more favorable results. The difference in outcomes was especially remarkable in the group with intermediate-risk cytogenetics (n = 397). These patients demonstrated significant differences in both OS (<5% blasts vs. ≥5% blasts = 59.3% vs. 34.0% p < 0.001) and RFS (<5% blasts vs. ≥5% blasts = 44.8% vs. 24.8%, p < 0.001). Among the patients with intermediate-risk cytogenetics, we analyzed 63 cases in which allogeneic stem cell transplantation was performed at the first remission. Patients with ≥5% blasts in the bone marrow demonstrated a significantly worse prognosis in terms of both OS and RFS, while no significant difference was observed in cumulative incidence rates (CIR) (2-year CIR 12.8% vs. 23.8%, p = 0.575). Among patients who required two courses of induction therapy at first remission (n = 24), non-relapse mortality occurred frequently. Furthermore, multivariate analysis of factors affecting OS and RFS revealed that <5% blasts in bone marrow was an independent prognostic factor (HR 1.92 [1.49-2.48]) p < 0.001, HR 1.66 [1.24-2.23] p < 0.001 respectively).
Conclusion
As an early assessment of bone marrow following remission induction therapy, having <5% of blasts in the bone marrow on day 15 was linked to not only a short-term remission rate, but also to long-term prognosis in terms of OS and RFS. A strong link to prognosis was observed particularly among patients with intermediate-risk cytogenetics; the group with ≥5% blasts had significantly worse outcomes even after allogeneic transplantation in the first remission. No significant difference in CIR was observed between the two groups following allogeneic transplantation during the first remission, but the non-relapse mortality rate was suggested to yield a difference in prognosis.
Fujita:Chugai Pharmaceutical CO.,LTD.: Honoraria. Kiyoi:Novartis Pharma K.K.: Research Funding; JCR Pharmaceutlcals Co.,Ltd.: Research Funding; FUJIFILM Corporation: Patents & Royalties, Research Funding; Zenyaku Kogyo Co., Ltd.: Research Funding; Phizer Japan Inc.: Research Funding; Toyama Chemikal Co.,Ltd.: Research Funding; AlexionpharmaLLC.: Research Funding; Celgene Corporation: Consultancy; Nippon Shinyaku Co., Ltd.: Research Funding; Alexion Pharmaceuticals: Research Funding; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; Takeda Pharmaceutical Co., Ltd.: Research Funding; MSD K.K.: Research Funding; Kyowa Hakko Kirin Co., Ltd.: Research Funding; Chugai Pharmaceutical Co., Ltd.: Research Funding; Mochida Pharmaceutical Co., Ltd.: Research Funding; Nippon Boehringer Ingelheim Co., Ltd.: Research Funding; Astellas Pharma Inc.: Consultancy, Research Funding; Eisai Co., Ltd.: Research Funding; Yakult Honsha Co.,Ltd.: Research Funding. Naoe:Amgen Astellas BioPharma K.K.: Honoraria; Fujifilm Corporation: Honoraria, Patents & Royalties, Research Funding; Sumitomo Dainippon Pharma Co.,Ltd.: Honoraria, Research Funding; TOYAMA CHEMICAL CO.,LTD.: Research Funding; Pfizer Inc.: Research Funding; CMIC Co., Ltd.: Research Funding; Astellas Pharma Inc.: Research Funding; Nippon Boehringer Ingelheim Co., Ltd.: Honoraria, Research Funding; Celgene K.K.: Honoraria, Research Funding; Chugai Pharmaceutical Co.,LTD.: Honoraria, Patents & Royalties; Bristol-Myers Squibb: Honoraria; Kyowa-Hakko Kirin Co.,Ltd.: Honoraria, Patents & Royalties, Research Funding; Otsuka Pharmaceutical Co.,Ltd.: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.