Abstract
FLT3 ITD mutations occur in both cytogenetically normal and abnormal AML. FLT3 ITD mutated AML is associated with genomic instability and clonal evolution (Gourdinet al., Cancer Genet 2014). The most common other molecular abnormality in FLT3 ITD AML, NPM1, has been shown to participate in DNA damage response and base excision repair but its role in clonal evolution in AML is poorly characterized (Polettoet al., Mol Biol of Cell 2014). We set out to describe the characteristics of clonal evolution in FLT3 ITD AML cases treated at Johns Hopkins Hospital (JHH) over the past 15 years to characterize clinical outcomes in these leukemias.
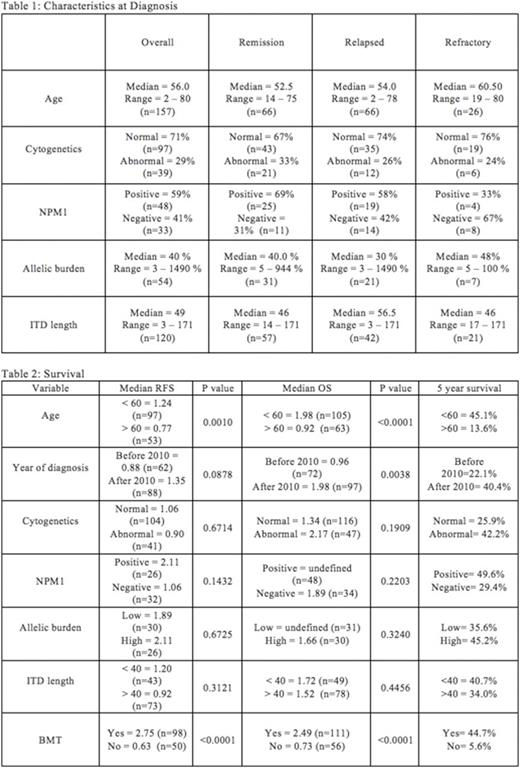
211 patients with FLT3 ITD AML were seen at JHH between 2000 and 2015, including newly diagnosed cases, and those referred for relapsed disease, with diagnostic and response information was available for 162 patients. 132 patients (81.4%) achieved remission, of which 66 subsequently relapsed (50%). 26 (16%) patients were refractory to therapy, 3 (1.8%) patients died during induction therapy, and 1 (0.6%) patient chose supportive care only. Characteristics at diagnosis are described in Table 1.
Median relapse free survival was 1.07 year and median overall survival was 1.48 years (Table 2). Relapse free survival (RFS) and overall survival (OS) were longer for patients who were less than 60 years old (p=0.001 and p<0.0001 Log-rank test). Overall survival was longer for patients who were diagnosed in or after the year 2010 (p=0.0038). Patients who received a BMT had the longest relapse free (median 2.75yr, v 0.63 yr, p=<0.0001) and overall survivals (median 2.49 v 0.73 yrs, p<0.0001). RFS and OS did not appear to be dependent upon ITD length, cytogenetics, or allelic burden. However, the 5-year survival for the low allelic burden (<40 bp) patients was 35.6%, compared to 45.2% for the high allelic burden patients (>/=40 bp). Median RFS was nearly twice as long for patients with NPM1 mutations (median 2.11 v 1.06 yrs, p=NS). The 5-year overall survival for the NPM1 positive patients was 49.6%, when compared to 29.4% for NPM1 negative patients.
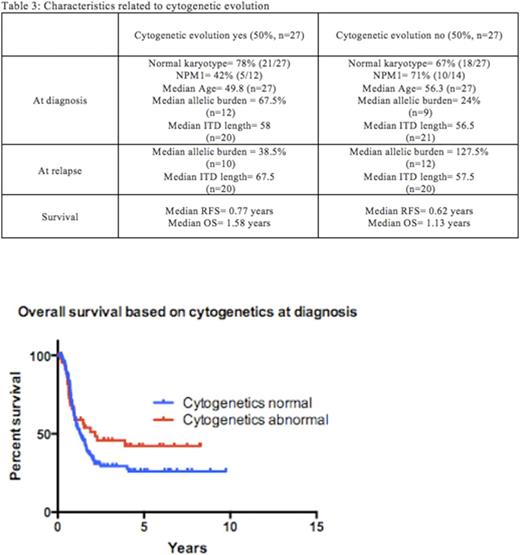
The median allelic burden and ITD length at relapse (49% and 62bp) were similar to those at diagnosis (30% and 56.5bp) and most remained FLT3 ITD mutated at relapse (88%)(Table 3). 50% of relapsed patients were found to have new cytogenetic abnormalities (27/54). Of those with new findings, 26/27 (96%) of those cytogenetic changes were acquired structural abnormalities (26/27). 9/27 (33%) were acquired numerical abnormalities and 8/27 (30%) acquired both structural and numerical abnormalities. Those patients who acquired structural abnormalities had a median RFS of 0.77 years versus those who remained cytogenetically unchanged from diagnosis had a median RFS of 0.62 years(p=NS).
Interestingly, patients with abnormal cytogenetics at diagnosis were not statistically more likely to acquire new cytogenetic abnormalities at relapse than patients with normal cytogenetics at diagnosis who relapsed (40% versus 54%, p=NS). Patients with NPM1 mutations at diagnosis appeared to acquire new cytogenetic abnormalities at relapse less often (5/15) than NPM1 negative patients (7/11)(33% versus 64%, p=NS), although this observation did not reach statistical significance (Fishers exact test). Patients who acquired cytogenetic abnormalities had a higher median allelic burden at diagnosis (67.5% versus 24%), although those without cytogenetic evolution had higher allelic burden at relapse (127.5% vs 38.5% median allelic burden). ITD lengths were similar for both groups at diagnosis (58bp and 56.5bp) but patients who acquired cytogenetic abnormalities also had longer median ITD lengths at relapse (67.5 bp versus 57.5 bp).
These results suggest that FLT3 ITD AML is a heterogeneous disease with variable clinical outcome linked to age at diagnosis, NPM1 status, FLT3 ITD allelic burden, and transplantation status. Cytogenetic clonal evolution is common in FLT3 ITD AML, irrespective of karyotype at diagnosis and likely reflects ongoing DNA repair and replication deficiencies in the leukemic clone. Further characterization of this deficiency may reveal future therapeutic targets.
Smith:Celgene: Consultancy, Other: member of DSMB. Levis:Novartis: Consultancy, Honoraria, Research Funding; Millennium: Consultancy, Research Funding; Daiichi-Sankyo: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.