Abstract
BACKGROUND: Follicular lymphoma (FL) is an indolent form of Non-Hodgkin B cell lymphoma that remains incurable with present therapies. Derived from germinal center B cells, FL B cells experience ongoing hypermutation of the immunoglobulin variable region gene. In addition, Michael Green, et al (PNAS; 2015), reported the presence of numerous somatic mutations to include those of the chromatin-modifying genes. These mutations accumulate over the course of the disease and play an important role in regulating gene transcription, B cell development and immune interactions.
Furthermore, FL tumors maintain a resemblance to primary lymphoid follicles, and as such, present with a number of infiltrating immune cells, especially T cells, the numbers of which vary from patient to patient. The close association and interaction of these immune cells with the tumor B cells play an important part in determining the disease biology (Dave SS, et al. N Engl J Med; 2004). For instance, tumor B cells, through cell-cell contact with these immune cells and/or through secretion of inhibitory cytokines such as TGF-b and IL-10, induce T cell exhaustion and apoptosis as well as suppressive T cell phenotypes (FoxP3+ T Regulatory cells) thus evading immune eradication (Yang Z-Z, et al. Blood 2007 and Ai WZ, et al. IntJ Cancer; 2009). They also promote their own survival and proliferation through their interaction with resident T follicular helper cells via CD40L/CD40 interactions (Ame'-Thomas P, et al. Blood; 2005).
As a corollary to an ongoing clinical trial, we received fine needle aspirates (FNAs) of easily accessible tumors from 14 patients with FL prior to any treatment. 6 of these patients had samples taken from a second site simultaneously. All samples were processed within 24 hours into a single-cell suspension; red blood cells were lysed. Cells were then stained with antibodies to delineate T, B, NK, dendritic, and myeloid cells, as well as their subsets. Antibodies against activation antigens, T cell exhaustion, inhibition and function were also used to characterize these cells. Finally, the cells were run on a 17-parameter LSRII (Becton Dickinson) and data analyzed via Cytobank, a web-based data storage and analysis tool.
PURPOSE: To better understand the biology of FL as represented by protein expression by the tumor cells and the immune cells that make up the microenvironment. We will especially look to evaluate the heterogeneity inherent in FL by flow cytometry across patients as well as within any one individual.
RESULTS: Each sample is stained with 4 panels of antibodies, 13 antibodies each, allowing us to measure over 100 cell subsets. A quick preview of all data shows that there is a high variability between patients in the percentage of T cells within the microenvironment (37.7% + 16.6% of all cells collected from all samples). This variability is represented by the differences in the CD4 T cell compartment (27.6 + 12.9%) and to a lesser degree in the CD8 compartment (7.7 + 3.7%). To note, this variability in T cells does not correlate with time from diagnosis to sample collection which ranged from 3.4 years to approximately 5 months. Also, this is in contrast to the similar percentage of CD4 and CD8 T cells expressing PD-1 (55.5 + 8.8% and 46.0 + 8.9%, respectively) across patients.
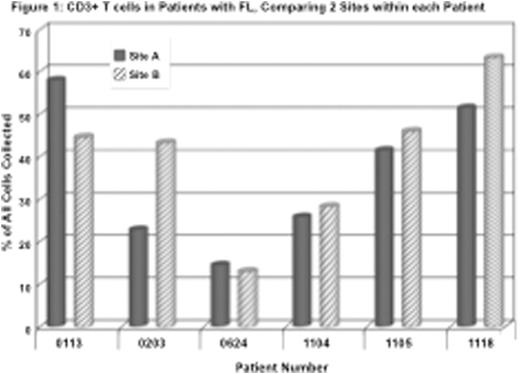
Notably, there is much less variability from site to site within each patient then between patients as demonstrated by Figure 1 where Site A and Site B are 2 separate lesions within each patient listed, sampled at the same time. Since FL presumably begins in a single site in the body and then becomes disseminated, the fact that a characteristic relationship exists between tumor cells and immune cells wherever the disease is found implies a mutual interdependence of the tumor cells in each case and their immune host component.
CONCLUSION: Follicular lymphoma is a very heterogeneous disease as would be expected by the diversity of mutations seen at the genomic level. This heterogeneity is also apparent in the microenvironment from one patient to another. Conversely, different tumor sites within each patient have a characteristic and fixed relationship to their immune microenvironment. The emergence of novel therapies for FL, including checkpoint antibodies such as anti-PD-1 and anti-PD-L1 and small molecules such as Ibrutinib, will be informed by understanding the differences as well as the similarities in each case of FL.
Levy:Kite Pharma: Consultancy; Five Prime Therapeutics: Consultancy; Innate Pharma: Consultancy; Beigene: Consultancy; Corvus: Consultancy; Dynavax: Research Funding; Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.