Abstract
Introduction: The outcome of MCL patients (pts) has improved over the last three decades, although this is debated outside clinical trials (Chandran, Leuk Lymphoma Aug 2012; Smith, Br J Cancer. April 2015). The Mantle Cell International Prognostic Index (MIPI) (Hoster, Blood Jan 2008) is based on 4 variables which predict survival: age (host factor), PS (tumor/host), LDH (tumor burden) and WBC (leukemic phase). The additional value of including co-morbidities into risk stratification has not been fully explored.
Methods: Using the COTA database we retrospectively analyzed MCL cases treated at John Theurer Cancer Center and the affiliated practices of Regional Cancer Care Associates from 2004 to 2016. Clinical and treatment characteristics, including calculation of the CCI index (Charlson J Chronic Diseases 1987) were captured via the COTA platform by extracting data from the electronic health records.
Results: 490 pts with MCL were evaluated and full longitudinal data from diagnosis is currently reported on 195 subjects. Pts characteristics included: male (66.15%), med age (65, range 34-94), stage IV (87%), LDH (elevated 26%; median 197, range 112-7950), MIPI (low 38%, interm 32%, high 30%), Ki-67 (≥30%: 51%, 86 NA), blastoid variant (16%, 35 NA), SOX-11 positive (87%, 143 NA), 17p abnormalities (p53 del or overexpression/mutation (24%, 88 NA) and b-2 microglobulin (b-2m) > 3 mg/L (52%). Frontline therapy consisted of R-Hyper-CVAD (with or without bortezomib on study) (36%), R-HyperCVAD or R-CHOP followed by high-dose therapy followed by autologous stem cell transplantation (ASCT) (9%), BR alone (8%), BR+ maintenance (8%), R-CHOP alone (4%), Rituximab (3%), R-BAC (3%), BR+ Ibrutinib vs placebo (2%), radiation (2%), R-Lenalidomide (1%), R-CHOP + maintenance (1%), other treatments (10%) while 10% of patients were treated expectantly Seventeen pts underwent ASCT consolidation (15 auto vs 2 allo (del17p/blastoid at presentation). Overall and progression free survival was computed using Kaplan Meier curves and significance tested using log-rank tests. The 5y OS for this entire cohort was 81. Overall, dose-intensive strategies (with or without ASCT) approach was associated with a 23 mo difference in PFS (median 74 mo, range 0-111 mo (intensive) vs median 51 mo, range 2-57 mo for the non-intensive group (p=0.37). The median OS was not reached for either group, with a 5y OS of 88% in intensive vs. 71% non-intensive regimens (p=0.14).
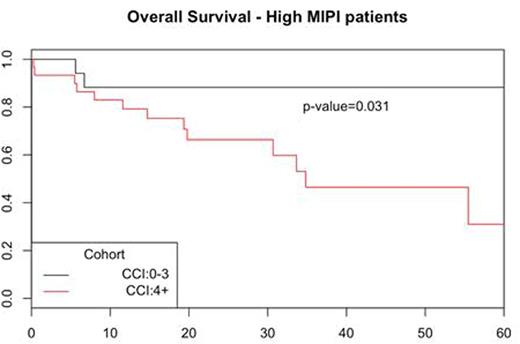
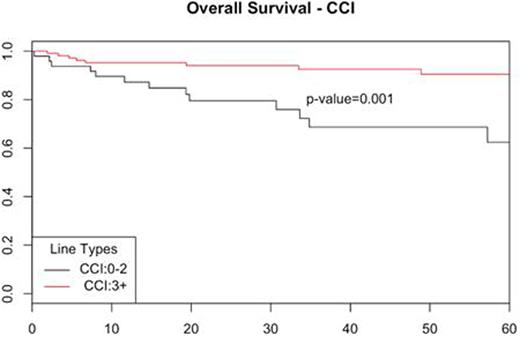
Using MIPI as stratification, low/intermediate risk pts had similar outcome in intensive and non-intensive therapy groups (5y OS 88% vs 71%; p=0.13). Pts with high MIPI had a 5 year OS of 80% in the intensive therapy group vs 46% for non-intensive group (p=0.376). The CCI scores for the whole cohort were 0 in 16 pts (8%), 1-3 in 81 pts (41%), and ≥ 4 in 98 pts (50%). Baseline CCI score (pre-treatment) was highly predictive of outcome with a 5y OS of 90% in CCI 0-3 vs 62% in CCI 3+ (p=0.001) (Figure 1). CCI did not predict complete response rate (CR) to induction therapy (CCI 0-3 94% vs CCI 3+ 80%). The median MIPI scores were 5.7 for CCI 0-3 and 6.3 for CCI 3+. Age is a component of both indexes but more heavily weighted in the CCI. Adding CCI to MIPI defined a subset of pts among the high MIPI group who did better than expected with a 5y OS of 88% in combined high MIPI / CCI 0-3 vs 31% for high MIPI / CCI 4+/ (p=0.03). b-2m (cut-off 3mg/L) correlated with 5y OS 93% vs 80%; (p=0.04) as previously reported but did not add to MIPI or CCI risk stratification. Ki-67 (30% cut-off) was marginally associated with OS: 5-y 89% vs 77% (p=0.06).
Conclusions: Our cohort is consistent with the improvement of MCL outcome comparing to historical controls and illustrates the importance of comorbidities captured at baseline. A combined CCI-MIPI approach might help identify pts who can still benefit from current therapy approaches in spite of age. Among the high MIPI score group, CCI further refines cohorts with significantly different outcomes.
Goy:Acerta: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Other: Research funding for clinical trials through institution; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; COTA: Membership on an entity's Board of Directors or advisory committees; Janssen/Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research funding for clinical trials through institution, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research funding for clinical trials through institution. Wu:COTA: Employment. Hansen:COTA: Employment. Arunajadai:COTA: Employment. Protomastro:COTA: Employment. Valentinetti:COTA: Employment. Murphy:COTA: Employment. Smith:COTA: Employment. Pe Benito:COTA: Employment. Hasan:COTA: Employment. Suryadevara:COTA: Employment. Feldman:Seattle Genetics: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Pharmacyclics: Speakers Bureau; Celgene: Speakers Bureau. Skarbnik:Pharmacyclics: Consultancy; Genentech: Speakers Bureau; Seattle Genetics: Speakers Bureau; Gilead Sciences: Speakers Bureau; Abbvie: Consultancy. Leslie:Celgene: Speakers Bureau; Seattle Genetics: Speakers Bureau. Pecora:COTA: Employment, Equity Ownership. Goldberg:Bristol Myers Squibb, Novartis: Speakers Bureau; Neostem: Equity Ownership; Novartis: Consultancy; COTA Inc: Employment; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.