Abstract
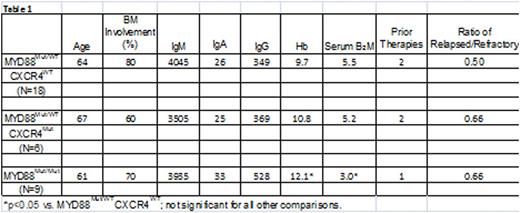
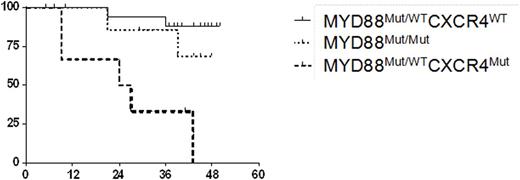
Activating somatic mutations in MYD88 and CXCR4 are present in 90-95% and 30-40% of untreated WM patients, respectively. Nearly all MYD88 somatic mutations involve a single nucleotide mutation that results in a change from leucine to proline at amino acid position 265, and most are heterozygous. In approximately 10-12% of untreated WM patients, MYD88 L265P is homozygous due to an acquired uniparenteral disomy (aUPD) or copy number alterations (Treon et al, NEJM 2012; Poulain et al, Blood 2013). Over 30 different types of nonsense and frameshift mutations in CXCR4 have been described in WM, and almost always are associated with mutated MYD88 (Hunter et al, Blood 2014). Mutated MYD88 activates BTK, and the BTK inhibitor ibrutinib is highly active in WM (Yang et al, Blood 2013; Treon et al NEJM 2015; Dimopoulos et al, ASH 2015). MYD88 mutations predict for response, while CXCR4 mutations are associated with slower response kinetics and lower response rates among mutated MYD88 WM patients on ibrutinib (Treon et al, NEJM 2015). In this study, we sought to clarify the impact of MYD88 homozygosity on ibrutinib activity in WM. We evaluated 33 previously treated, symptomatic WM patients who received ibrutinib on a clinical trial (NCT01614821), and for whom baseline and serial bone marrow (BM) CD19-selected cells were available for sequencing, copy number (CNA) and aUPD analysis. Mutated MYD88 homozygosity was determined by establishing the ratio of mutant (Mut) versus wild-type (WT) allele expression by Sanger sequencing. CNA status was determined using TaqMan real-time PCR assays. For patients with unaltered copy number, the presence of aUPD was determined by analyzing the tumor/germline allele balance using established TaqMan genotyping assays. At baseline, 9/33 (27.3%) of patients were homozygous for mutated MYD88. Mutated MYD88 homozygosity was confirmed to be due to deletion of the wild-type MYD88 allele by CNA in one patient, and amplification of the mutant MYD88 allele in another patient. TaqMan genotyping assays confirmed the presence of an aUPD in 6 patients that were confined to Chr. 3p. CXCR4 mutations were highly prevalent (8/9; 88.8%) among mutated MYD88 homozygous versus heterozygous (7/24; 29.2%) patients (p=0.014). We next assessed the impact of mutated MYD88 zygosity and CXCR4 mutation status on ibrutinib treatment outcome. The baseline characteristics for patients based on mutated MYD88 zygosity and CXCR4 mutation status are shown in Table 1. Following ibrutinib treatment, serum IgM levels declined by -0.89%, -0.54%, and -0.61% among MYD88Mut/WTCXCR4WT, MYD88Mut/WTCXCR4Mut, and MYD88Mut/Mut patients, respectively. Hemoglobin levels improved by 40.5%, 16.2% and 21.6% among MYD88Mut/WTCXCR4WT, MYD88Mut/WTCXCR4Mut, and MYD88Mut/Mut patients, respectively. Major responses (PR or better) were observed in 100%, 50%, and 89% of MYD88Mut/WTCXCR4WT, MYD88Mut/WTCXCR4Mut, and MYD88Mut/Mut patients, respectively. VGPR occurred in 50% of MYD88Mut/WTCXCR4WT patients, and 16.7% and 22.2% of MYD88Mut/WTCXCR4Mut, and MYD88Mut/Mut patients, respectively. Progression-free survival was also impacted by mutated MYD88 zygosity and CXCR4 mutation status (Figure 1). Median PFS was not reached for patients with MYD88Mut/WTCXCR4WT and MYD88Mut/Mut, and was 25.5 months for those with a MYD88Mut/WTCXCR4Mut mutation status (Log-rank Chi-square: 14.74, p=0.0006).
Conclusions: MYD88 homozygosity is strongly associated with activating mutations in CXCR4, and appears to confer a better outcome for WM patients with CXCR4 mutations on ibrutinib therapy.
Treon:Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy. Castillo:Pharmacyclics: Honoraria; Abbvie: Research Funding; Otsuka: Consultancy; Biogen: Consultancy; Millennium: Research Funding; Janssen: Honoraria. Palomba:Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.