Abstract
Background: Waldenström macroglobulinemia (WM) is difficult to study in randomized clinical trials because of its rarity, and higher incidence among older patients (pts). The most recent published trial focusing on WM (Leblond et al., J Clin Oncol 2013) compared chlorambucil with fludarabine-treatments no longer in common use. Recent data (Olszewski et al., Oncologist 2016) show that over 80% of WM pts in the United States are now treated with rituximab (R) alone or in combination with chemotherapy, although the effect of R on overall survival (OS) in WM has not been shown in clinical trials (Buske et al., Leukemia, 2009). Our objective was to provide comparative evidence of therapeutic efficacy for R-based immunochemotherapy in WM by applying causal inference methods to population-based, observational data.
Methods: Using Medicare claims from 1999-2013, linked to the Surveillance, Epidemiology and End Results registry data, we identified WM patients (pts) >65 years old, who initiated first-line chemotherapy with or without R, with purine analogues (PUR, fludarabine or cladribine) and/or classic alkylating agents (ALK, chlorambucil or cyclophosphamide). Pts receiving both fludarabine and cyclophosphamide were grouped as PUR. Factors associated with treatment selection were studied in multivariable mixed-effects logistic models. We then conducted 3 separate comparative analyses: 1) regimens with R versus without R, 2) R monotherapy versus combination immunochemotherapy, and 3) PUR- versus ALK-based regimens. In each case, we balanced baseline characteristics (age, sex, race, socioeconomic status, comorbidities, performance status, time from diagnosis), and indicators of WM severity previously validated to correlate with OS (anemia, transfusions, plasmapheresis) using propensity score analysis, to minimize indication bias and simulate a randomized experiment. We then compared OS and select adverse events within 90 days of treatment (hospitalization, transfusion, or plasmapheresis) using survival or log-binomial models weighted by inverse probability of treatment (IPT), reporting hazard ratio (HR) or relative risk (RR) with 95% confidence intervals (CI).
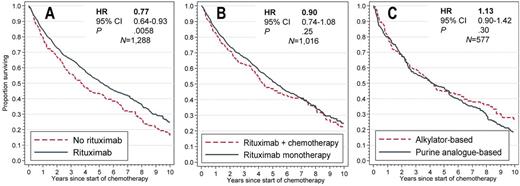
Results: Among 1,310 pts (median age 78 years, 43% women), 54% received first-line R monotherapy, 12% R+PUR, 12% R+ALK, 14% PUR-(without R)- and 8% ALK-(without R)-based regimen. Receipt of R was more likely among pts with metropolitan residence, diagnosis after 2003, and baseline neuropathy, and significantly varied by treating physician (intra-class correlation, 45%, CI 25-66%, P<.0001). Unadjusted 5-year OS was 42.0% (CI, 36.1-47.8) for chemotherapy without R, 51.9% (CI, 46.0-57.6) for chemotherapy with R, and 50.6% (CI, 46.4-54.7) for R alone.
In each comparative analysis, we achieved adequate balance of confounding variables using propensity scores. In the IPT-weighted outcome models, OS was significantly better for pts who received R as part of their therapy compared with those who did not (HR, 0.77; CI, 0.64-0.93; P=.0058; Fig. A), without difference in the studied toxicities. Within 3 months of starting therapy, there was an overall 20.4% frequency of transfusion, 9.6% of hospitalization, and 4.4% of plasmapheresis. The RR for plasmapheresis after R-based regimen was 1.09 (CI, 0.63-1.90, P=.76). There was no significant difference in OS between pts receiving R alone or in combination with chemotherapy (HR, 0.90, CI, 0.74-1.08, P=.25, Fig. B), but the risk of transfusions (RR, 0.71; CI, 0.56-0.88; P=.002) and hospitalizations (RR, 0.52; CI, 0.34-0.79; P=.002) was lower after single-agent R. Furthermore, there was no evident difference between PUR- or ALK-based regimens in OS (HR, 1.13, CI, 0.90-1.42, P=.30, Fig. C), or in the studied toxicities.
Conclusions: This population-based, comparative effectiveness study provides evidence of OS benefit of R (as monotherapy or combination immunochemotherapy) for Medicare beneficiaries (>65 years old) with WM. Toxicity of single-agent R was lower compared with combination regimens, without a difference in survival, thus confirming its utility as a treatment option for older pts who do not have a strong indication for cytotoxic therapy. Acknowledging likely pre-selection on the basis of (unrecorded) IgM levels, and possible uncaptured prophylactic measures, R-based therapy did not appear to result in a higher risk of plasmapheresis or hospitalization.
Olszewski:TG Therapeutics: Research Funding; Genentech: Research Funding; Bristol-Myers Squibb: Consultancy. Treon:Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy. Castillo:Janssen: Honoraria; Millennium: Research Funding; Pharmacyclics: Honoraria; Abbvie: Research Funding; Otsuka: Consultancy; Biogen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.