Abstract
Introduction: With approval of Daratumumab (Dara), targeting CD38 has become an attractive therapeutic option for various B cell cancers including WM. Using preclinical models of WM we investigated CD38 expression patterns, the impact of drug-resistance and the anti-WM effects of Dara, in vitro. An important aspect of our evaluation was the identification of optimal CD38 expression that correlates with maximal cytotoxic effects of Dara; such an approach has not been attempted previously in WM.
Methods: WM cell lines (BCWM.1, MWCL.1 and RPCI.WM1) with isogenic proteasome inhibitor-resistant (CR, resistant to carfilzomib, bortezomib and ixazomib) and ibrutinib-resistant subclones (IR) were used. CD38 expression was measured by flow cytometry and a grading system was developed based on increasing CD38 MFI values with 1+= <50,000, 2+ = >50,000 and 3+ = >100,000. Daratumumab-induced specific lysis due to antibody dependent cell cytotoxicity (ADCC) or complement dependent cytotoxicity (CDC) was measured using the Calcein AM assay at an effector to target ratio (E:T) of 100:1. Peripheral blood mononuclear cells (PBMCs) and complement-containing serum were obtained from healthy human donors. Daratumumab, carfilzomib and ibrutinib were obtained from commercial sources.
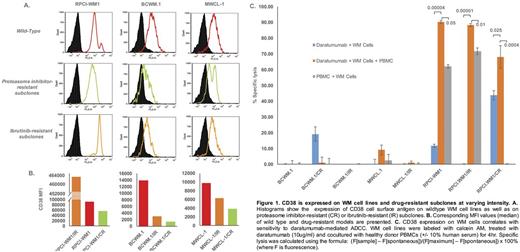
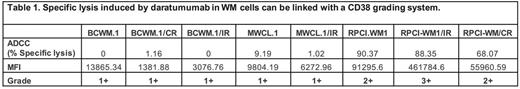
Results: Expression of CD38 (MFI) was highest in RPCI-WM1 (91295.6) > BCWM.1 (13865.3) > MWCL-1 (9804.19) (Figure 1A.). Intriguingly we observed an interesting change in CD38 expression with induction of drug resistance; all CR clones showed a decrease in MFI (RPCI-WM1/CR; øMFI 55960.5 > MWCL-1/CR; øMFI 3890.0 > BCWM.1/CR; øMFI 1381), while decreased expression was noted in all IR clones except one (MWCL-1/IR; øMFI 6272.96, BCWM.1/IR; øMFI 3076 vs. RPCI-WM1/IR; _MFI 461784.6) (Figure 1B). When we applied our CD38 expression grading system, RPCI-WM1/IR cells classified as Grade 3+, RPCI-WM1 and its CR clones as Grade 2+, while all other WM cells were Grade 1+. We noted that utilization of this grading system was helpful in correlating the extent of cytotoxicity to the level of CD38 expression (Table 1). We next treated WM cells with Dara and observed direct lysis in only RPCI-WM1/CR (43%) > BCWM.1/CR (19%) and RPCI-WM1 (11%) cells. However, when Dara-treated WM cells were cocultured with PBMCs, significantly increased ADCC was noted in RPCI-WM1 (90.3%), RPCI-WM1/IR (88.3%) and RPCI-WM1/CR (68.0%) cells (Figure 1C). Although CDC has been reported as a mechanism of mAb-mediated cell death in WM our evaluation of CDC as a potential effector mechanism in daratumumab-treated WM was negative as expected, highlighting ADCC rather than CDC as the primary cytotoxic mechanism.
Conclusion: Our analysis of CD38 in WM preclinical models highlights several important findings: 1.) Expression of CD38 changes as WM tumor clones evolve with induction of drug-resistance; an effect that may also be cell-type specific as evidenced by increased CD38 expression in RPCI-WM1/IR cells, 2.) Daratumumab can exert a direct lethal effect in WM cells but this effect is variable and cell line dependent, 3.) Expression of CD38 is an important factor directing cytotoxicity of Dara however antigen density appears to be an important factor and a grading system can be beneficial for future preclinical and/or clinical studies, 4.) ADCC and not CDC appear to be the prominent mechanism of action and 5.) ADCC-mediated cytolytic activity of Dara is contingent on CD38 expression as evidenced by the most specific lysis being noted in CD38 Grade 2+ and 3+ expressing WM cells (Table 1.). These observations can help direct optimal design of clinical studies with Dara in WM where drug resistance and CD38 expression should be further explored as factors contributing to the clinical efficacy of this new agent.
Ailawadhi:Pharmacyclics: Consultancy; Novartis: Consultancy; Amgen Inc: Consultancy; Takeda Oncology: Consultancy. Ansell:BMS, Seattle Genetics, Merck, Celldex and Affimed: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.