Abstract
Introduction:
The initial approach to elderly patients with Diffuse Large B-cell lymphoma (DLBCL) is usually based on the subjective judgment of the physician on the individual patient's ability to tolerate treatment with curative intent. "Comprehensive Geriatric Assessment" (CGA) is based on the use of the ADL (Activity of Daily Living), IADL (Instrumental ADL) and CIRS-G (Comorbidity Index Rating Scale for Geriatrics) scales and represents a tool to standardize initial patients fitness assessment and for planning systemic therapy. So far CGA has been rarely used in prospective studies and lacks a formal validation in patients with lymphoma.
Objectives:
FIL is conducting a prospective study with the aim of validating the use of CGA on a large series of elderly patients with DLBCL and to test a CGA based approach to the patient. CGA results will be used to define treatment goals that are the cure for the FIT subjects, and palliation for the FRAILs. Treatment goal for the UNFIT is cure with less toxic regimens.
Methods:
This study is conducted using a web based platform, accessible from the reserved area of the FIL website, to perform a quick and objective CGA evaluation of consecutive patients ≥ 65 years with untreated DLBCL. Patients younger than 80 years, without impairment of ADL and IADL and without severe comorbidities were considered FIT; those with intermediate fragility or those older than 80 years with FIT profile were classified as UNFIT (UN); those with severe impairment of ADL, IADL and CIRS and those older than 80 years with an UN profile were classified as FRAIL (FR). Informed consent was required to enrol patients in this study; the planned sample size was 1000 patients.
Results:
The study started in December 2013. At time of current analysis 792 patients have been registered by 45 centres: 328 (41%), 207 (26%), and 257 (33%) were classified as FIT, UN and FR, respectively.
Median age was 77 years (yrs) (65-95); 73 (65-79), 80 (65-95) and 81 (65-95) yrs for FIT, UN and FR patients respectively; overall 65% were in stage III-IV. By univariate analysis, the three categories differed in terms of median age (p<0.001), B-symptoms (p=0.035), ECOG PS>1 (p<0.001), elevated LDH (p=0.035) and IPI score (p=0.004).
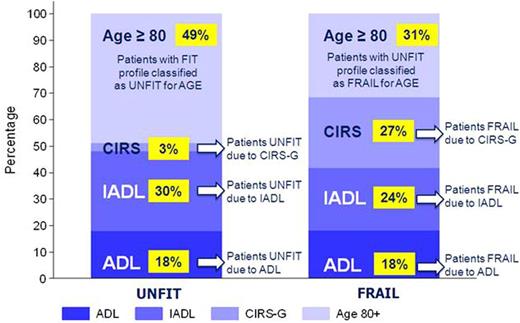
Fourty-nine percent of cases were defined as UN only because of age (≥80 yrs); other reasons for UN were impairment of IADL, ADL in 30% and 18%, respectively. Only 3% of cases were UN due to CIRS (5-8 comorbidities of grade 2). Regarding FRAIL patients 27% of patients were classified in this group due to CIRS impairment, 24% and 18% due to IADL and ADL impairment, respectively. In remaining 31%, patients were FRAIL due to age ≥80 yrs (Figure 1). The most frequent altered ADL items among UN and FR patients were continence (15%) and bathing (30%), respectively; regarding IADL the most frequent altered items were buying and food preparation for either UN (19% and 12%) and FR (53% and 41%). Most frequent grade 3 comorbidities among the 257 FR patients were those referred to heart (16%), vascular system (9%), muscoloskelatal (7%), and genitourinary (5%).
Data on planned treatment were available in 643 patients. Rituximab was used in all but 6 (2%), 18 (12%), and 35 (17%) FIT, UN, and FR cases.
Treatment with curative intent (full doses R-CHOP-like regimens) was used in 94% and 65% of FIT and UNFIT cases; surprisingly curative intent was declared also in 38% of FRAIL cases. Six percent, 25% and 26% of FIT, UN and FR patients were treated with an attenuated R-CHOP-like immuno-chemotherapy regimens.
Palliative regimens were used in 36% and 10% of FR and UN patients respectively.
Conclusion:
The preliminary data of Elderly Project showed that, with the CGA criteria as adopted in this study, the 59% of elderly patients with DLBCL at diagnosis were not FIT.
Rituximab and doxorubicin containing regimen seems to be the reference treatment for FIT, UN and also for a significant proportion of FR patients but the actual value of using this approach for non-FIT patients is not clear and will be assessed with this project.
Contribution of Activity of Daily Living (ADL), Instrumental ADL (IADL), Comorbidity Index Rating Scale for Geriatrics (CIRS-G) scales and Age to Fitness Status
Contribution of Activity of Daily Living (ADL), Instrumental ADL (IADL), Comorbidity Index Rating Scale for Geriatrics (CIRS-G) scales and Age to Fitness Status
Merli:Teva Pharmaceuticals Industries: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees. Luminari:Roche: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accomodations, Expenses; Takeda: Other: Travel, Accomodations, Expenses; Teva Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria. Cavallo:JANSSEN: Honoraria; CELGENE: Honoraria; ONYX: Honoraria. Chiappella:Roche: Speakers Bureau; Amgen: Speakers Bureau; Pfizer: Speakers Bureau; Teva: Speakers Bureau; Janssen-Cilag: Speakers Bureau; Celgene: Speakers Bureau. Spina:Mundipharma: Membership on an entity's Board of Directors or advisory committees, Other: Speaker Fee; Teva Pharmaceuticals Industries: Membership on an entity's Board of Directors or advisory committees, Other: Speaker Fee.
Author notes
Asterisk with author names denotes non-ASH members.