Abstract
Objectives Explore patients' and physicians' concerns regarding tyrosine kinase-inhibitor (TKI)-therapy in chronic myeloid leukemia (CML). Identify socio-demographic and clinical variables associated with patients' concerns.
Methods Between September, 2015 and June, 2016, an anonymous non-interventional, cross-sectional multiple choice questionnaire including 16 common issues related to TKI-therapy of CML were distributed to adults with CML receiving TKI therapy for 3 months and to physicians in medical meetings. Responses were tabulated and analyzed.
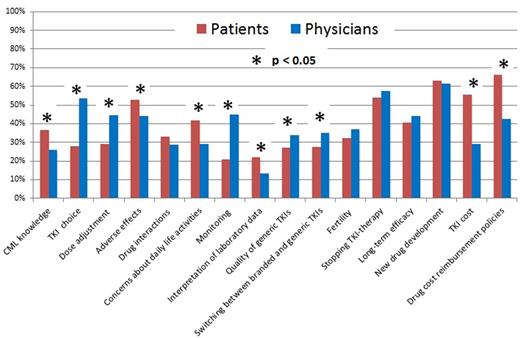
Results We analyzed evaluable questionnaires from 1,427 persons with CML, 1,384 of whom (97%) were in the chronic phase. 877 (62%) were male. Median age was 42 years (range, 18-88 years). 738 (52%) had a bachelor degree. 1,163 (82%) started TKI-therapy <6 months after diagnosis and 1,066 (75%) were receiving imatinib when they answered the questionnaire. 941 (66%) were receiving branded TKIs including Glivec®, Tasigna® and Sprycel®. Median TKI-therapy duration was 29 months (range, 3-182 months). 1,086 (76%) had achieved a complete cytogenetic response (CCyR) and 572 (41%), a complete molecular response (CMR). 279 (20%) were receiving free TKIs whereas 1,143 (80%) paid some or all of their TKI costs. Median annual out-of-pocket TKI expense in payors was $10,800 USD (range, $250-$14,600 USD) compared with an average annual disposable incomes of rural ($1,800 USD) and urban ($4,800 USD) households in 2015 in China (http://www.stats.gov.cn/). 370 questionnaires were distributed to hematologists, 260 (70%) of which were returned and were analyzable. 174 physician respondents (67%) were from university or teaching hospitals and 157 (60%) were in senior positions. 154 (59%) hematologists treated >5 persons with CML per month. Median number of concerns regarding TKI-therapy was 5 (range, 0-16) for patients and physicians. The top 5 issues for both groups were: (1) drug cost and reimbursement policies; (2) new drug development; (3) stopping TKI-therapy; (4) adverse effects; and (5) long-term efficacy. 11 issues attracted proportionally differential attention between patients and physicians (Figure). Patients were more concerned with: (1) TKI-reimbursement policies; (2) TKI cost; (3) TKI-related adverse effects; (4) concerns about daily life activities; (5) CML knowledge; and (6) interpretation of laboratory data. Physicians were more concerned with: (1) TKI choice; (2) dose adjustment; (3) monitoring; (4) quality of generic TKIs; and (5) switching between branded and generic TKIs. Multivariate analysis showed female sex (OR=1.4; [1.1, 1.8]; p=0.005), bachelor degree (OR=1.6; [1.2, 2.0]; p<0.001), starting TKI-therapy 6 months from diagnosis (OR=1.4; [1.0, 1.9]; p=0.033), high out-of-pocket expenses (OR=1.3; [1.1, 1.4]; p<0.001), and severe adverse impact on work and daily life (OR=1.3; [1.1, 1.7]; p=0.011) were associated with greater concerns for patients. Female sex (OR=2.3; [1.4, 3.9]; p=0.002) and >50 CML patients treated per month (OR=7.9; [1.7, 36.9]; p=0.009) were associated with greater concerns for physicians.
Conclusions TKI reimbursement policies, new drug development, stopping TKIs, TKI-related side effects and long-term efficacy were common concerns of persons with CML and physicians. Haematologists should pay greater attention to patients' concerns regarding these variables. This may improve quality of care of persons with CML and improve patient-physician communication.
Patients' and physicians' concerns regarding TKI-therapy of CML
Patients' and physicians' concerns regarding TKI-therapy of CML
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.