Abstract
Background. Calreticulin (CALR) mutations are detected in the majority of JAK2 wild-type (wt) patients with Essential Thrombocythemia (ET) or Primary Myelofibrosis (MF). At variance with JAK2 and MPL point mutations, CALR mutations are highly heterogeneous, with several types of insdel reported to date, so that sequencing techniques are needed to cover the whole spectrum of mutations. CAL2 is a new monoclonal antibody specifically recognizing the C-neoterminal peptide derived from all the frameshift mutations of CALR.
Methods. We retrospectively analyzed 172 ET patients followed at our Institution from 1980 to 2015 who were tested for JAK2V617F at the time of diagnosis or during follow-up. In JAK2wt patients exon 9 CALR mutations were searched by PCR and capillary electrophoresis and MPLW515L/K by ARMS-PCR. In the same patients, bone marrow (BM) biopsies were immunostained with CAL2 and histologically reviewed for megakaryocytic features.
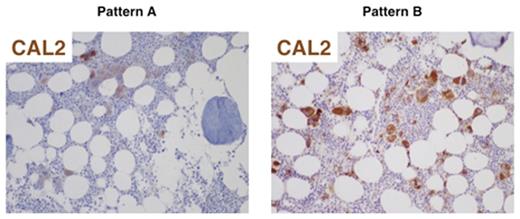
Results. Median age at diagnosis was 54 years (range 14-87) and median time from first evidence of thrombocytosis to BM biopsy was 8.4 months (range 0-175). According to driver mutations, patients were classified as JAK2V617F-mutated (n=119, 69%), MPL-mutated (n=2, 1%), CALR-mutated (n=31, 18%) or triple-negative (n=20, 12%). As expected, CALR-mutated patients had significantly higher platelet count than JAK2V617F (799 vs 657 x 109/L, respectively; p=.019) and lower hemoglobin values (mean 13.6 vs 14.5 g/dL, respectively; p=.003). At a median follow-up of 9.8 years, incidence of thrombosis was significantly lower in CALR- than in JAK2V617F-mutated patients (3% vs 21%, respectively; p=.017), while no differences were observed in clinical presentation and long-term outcome between CALR-mutated and triple-negative patients. Concordance between molecular and immunohistochemical (IHC) detection of CALR mutations was optimal (CohenÕs kappa >0.8) but not complete, since 3 patients were positive by IHC only and 1 patient was positive by molecular only. In CAL2-positive BM samples (n=30) we defined 2 patterns (figure), characterized by staining of megakaryocytes only (pattern A, 37%) or staining of megakaryocytes and myeloid precursors (pattern B, 63%). Type B biopsies tended to have higher median cellularity (48% vs 30%; p=.22), higher median megakaryocytic number for HMF (20 vs 12.5; p=.14), higher frequency of megakaryocytic clusters (100% vs 60%; p=.014) and higher frequency of grade-1 fibrosis (81% vs 60%; p=.37) with respect to type A samples. One type A patient and 2 type B patients progressed to post-ET MF or acute leukemia. Moreover, 3 patients had a BM biopsy performed at the time of MF evolution but not at diagnosis of ET, and they displayed a type B pattern.
Conclusions. CAL2 identifies CALR-mutated ET patients in a rapid, sensitive, specific and economic manner and it integrates molecular biology informations. Prognostic value of different IHC patterns should be confirmed in wider and indipendent series.
Immunohistochemical patterns of CAL2 staining in ET samples: megakaryocytes only (pattern A) or megakaryocytes and myeloid precursors (pattern B).
Immunohistochemical patterns of CAL2 staining in ET samples: megakaryocytes only (pattern A) or megakaryocytes and myeloid precursors (pattern B).
Bonifacio:Ariad Pharmaceuticals: Consultancy; Amgen: Consultancy; Bristol Myers Squibb: Consultancy; Pfizer: Consultancy; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.