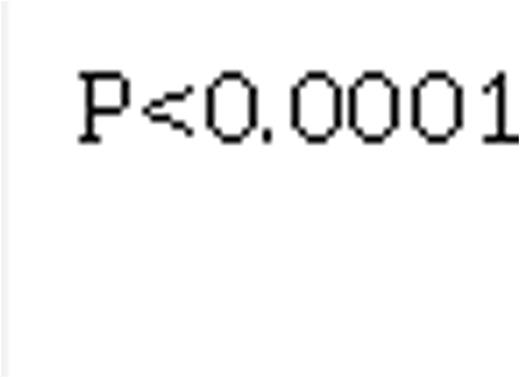Abstract
Background: Myelofibrosis (MF) is the less frequent Philadelphia-negative myeloproliferative neoplasms (MPN). We have included in a nationwide database primary (P), post-polycythemia (PPV) and post-essential thrombocythemia (PET) MF diagnosed in France since 2005.
Methods: Inclusion criteria were: diagnostic of MF after 2005; according strictly to WHO (bone marrow biopsy mandatory); informed consent. The registry was launched in Oct. 2013, and 26 hematology centers included patients (pts). Summary statistics were reported, namely median [Interquartile range, IQR] or percentage. Baseline characteristics were compared across IPSS groups using chi-square test or Mann and Whitney test. Kaplan-Meier survival curves were plotted and compared by the log-rank test.
Results: At time of analysis (June 2016) a total of 527 pts were included in the registry, complete baseline data were available in 499 (95%), and follow-up (FU) data in 433 (87%).
Median [IQR] age and M/F sex ratio were 71 [63-78] years and 315/184 (1.7), respectively (resp). 301 (60%) pts had PMF, 182 (36%) had secondary MF (including 64 PPVMF and 118 PETMF) and 16 had pre-fibrotic MF. Five percent of pts had a familial history of hematologic malignancy, and 22% a history of thrombosis or hemorrhage.
Splenomegaly was present in 386 (77%) with a median [IQR] spleen size of 4 cm [1-8] below costal margin, and was symptomatic in 11% of pts. Constitutional symptoms were present in 107 (21%) (weight loss in 56, night sweats in 61, fever in 14), and ECOG score was 0, 1, 2, and 3 in 53%, 36%, 11% and 0.3% of pts, resp.
Median [IQR] Hemoglobin, WBC and platelet counts were 109 g/L [94-122], 9.3 G/L [5.7-16.0] and 257 G/L [138-430], resp. Circulating blast cells were present in 41%, LDH was above normal value in 95% of pts, median EPO level was 54 [11 - 57] U/L. Grade of fibrosis (WHO) was 1, 2, and 3 in 2%, 66% and 32% of pts, resp. Karyotype was done in 321 pts, normal in 173 (54%), abnormal with favorable prognostic value in 89 (30%) and unfavorable in 30 (10%) (29 failures). A total of 461 (92%) pts had molecular testing: 60% were JAK2V617F positive, 4% had MPL and 7% had CALR mutations, and 99 (28%) over the 352 pts with triple testing were triple negative.
IPSS risk categories were low, int-1, int-2 and high in 68 (14%), 168 (34%), 158 (32%), and 105 (21%) pts, resp. In addition to constitutional symptoms, there was a significant increase in the prevalence of clinical signs across IPSS categories (from low to high risk): symptomatic spleen (p=0.016) -though no difference in spleen size was found (p=0.18)-, early satiety (p=0.013), ECOG score (p= 0.0001), bone pain (p=0.002). Moreover, among biological parameters, there was an increase across IPSS groups in WBC (p=0.029), LDH (p=0.0007), ferritin (p=0.003), circulating CD34+ cells (p=0.020), EPO level (p=0.035). In contrast, a decrease was seen for hemoglobin and platelets (p=0.0001 for both). Lastly, frequency of grade 3 fibrosis increased with IPSS (p=0.043), while no evidence of difference was found regarding abnormal karyotype and mutational pattern.
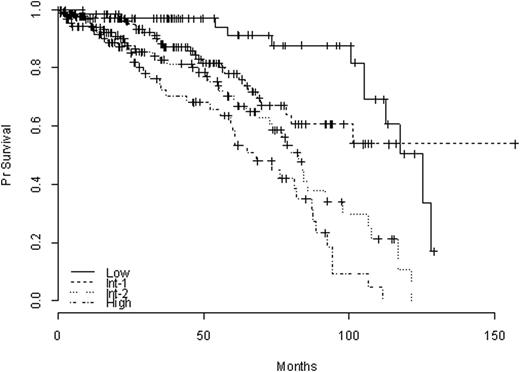
Median FU was 33 months [9-63 months]. Among those 433 pts with FU data, median FU was 38 months [19-68], and 124 (29 %) pts had died at the time of analysis, including 13%, 17%, 31%, and 40% of pts from the Low, Int-1, Int-2, and High risk groups, resp (p= 0.0001, figure 1).
In the 450 pts with treatment data, treatments received during FU included cytoreductive drug (41%), Jak-inhibitors (35%), Interferon alpha (18%), IMIDs (4%). Splenectomy was performed in only 14 (3%) pts. Forty percent of pts received packed red blood cells, and 12% platelets transfusions. 49 (11%) patients participated in a clinical trial, and 27 (6%) were allografted.
Conclusion:
This is the first analysis of the French MF observatory after inclusion of more than 500 pts diagnosed and treated during the past 10 years. Complete baseline data and follow-up information available for the majority of pts should allow for new studies of outcomes and influence of clinical and biological parameters, as well as reassessment of prognostic models in the era of new targeted therapies.
Etienne:Pfizer: Speakers Bureau; BMS: Speakers Bureau; ARIAD: Speakers Bureau; novartis: Consultancy, Speakers Bureau. Tavitian:Novartis: Membership on an entity's Board of Directors or advisory committees. Ugo:Novartis: Membership on an entity's Board of Directors or advisory committees. Kiladjian:Novartis: Honoraria, Research Funding; AOP Orphan: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.