Abstract
Introduction:The selectiveBruton's tyrosine kinase (BTK) inhibitor GS-4059 (ONO-4509) showed remarkable efficacy and tolerability in a phase 1 multicenter dose escalation study (NCT01659255) in 28 patients with relapsed and refractory (R/R) CLL (Walter et. al. Blood 2016). No maximum tolerated dose was identified in the CLL cohort andintrasubject dose escalation was allowed. Patients discontinued study participation due to disease progression (3), adverse events (2), death (3), and investigator decision (1). Nineteen patients whocontinued to respond or derive clinical benefit from GS-4059 enrolled in the extension study (NCT02457559) in December 2015. Theupdated survival and safety data are presented here.
Methods:Nine dose cohorts ranged from 20 mg OD to 600 mg OD and 300 mg BID. Patients were evaluated clinically every 4 weeks; radiographic evaluation was at or prior to enrolment, at 12 weeks, and every 24 weeks thereafter.Targeted next-generation sequencing using a custom array (SiestmasGenomicos, Valencia, Spain) was used to detect mutations in 36 CLL-related actionable genes for all 28 patients with CLL enrolled in the phase 1 study. All mutations were confirmed by Sanger sequencing.
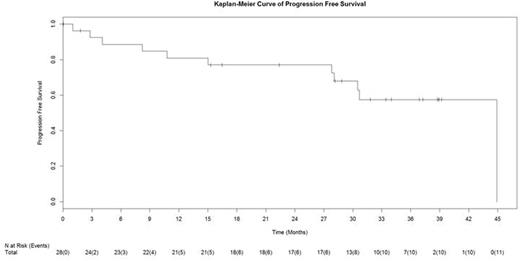
Results: As of June 1, 2016, 17 patients remain on study. Two patients discontinued study treatment, 1 due to idiopathic thrombocytopenic purpura and 1 due to progressive disease (dose 20 mg OD). The overall median duration of treatment is 799 days with a median progression-free survival of 45 months (Figure 1). There was no improvement in overall response over the course of the first 6 months of the extension study. Further updated safety analysis has not identified any new late-occurring toxicity or any reports of Richter's transformation. Bleeding-related adverse events (AEs) have all been grades 1-3. The incidence of bleeding-related events in the long-term extension study has been significantly less than in the initial 6 months of treatment (11% vs 68%). Overall,46% of patients had a maximal weight gain of grade 1-3 on study (grade 1 [5], grade 2 [3], grade 3 [5]). Maximal weight loss of grade 1-2 on study was noted in 36% of patients (grade 1 [8], grade 2 [2]). No dose reductions for toxicity have been required.
Depth and duration of response were independent of interphase FISH abnormalities, mutational profile and response to last treatment prior to study entry. Nine of 28 patients initially enrolled had CLL with TP53 mutations in the presence of a TP53 deletion; 2 cases of mutation occurred independently of TP53 loss. Seven NOTCH1, 8 SF3B1, and1 POT1 mutations were identified. We did not identify a genetic subset of patients who appeared at risk of early progression. In total, 7 of 9 patients with TP53 mutation remain on therapy; 1 patient progressed and 1 died from sepsis. One patient with TP53, NOTCH1, and SF3B1 mutations has been on therapy for 1100 days, on a dose of 40 mg OD. Another patient with a TP53 mutation who progressed on 40 mg OD (March 2015) but lacked BTK and PLCG2 mutations, has achieved a partial response while on 600 mg OD dose. A second lymphocytosis similar in magnitude and duration to that seen with the initial treatment with GS-4059 was observed on dose escalation.
Conclusion: GS-4059 continues to demonstrate preliminary efficacy and tolerability in the treatment of CLL, irrespective of cytogenetics or mutational profile. The response rate and depth of response in CLL are comparable to the results reported for other BTK inhibitors, and long-term treatment discontinuation rates due to AEs are low.
Updated Progression-Free Survival Curve for CLL Patients.
Updated Progression-Free Survival Curve for CLL Patients.
Walter:Gilead Sciences: Other: Contribution to travel expenses to conference. Cartron:Roche: Consultancy, Honoraria; Celgene: Honoraria; Gilead: Honoraria; Jansen: Honoraria. Morschhauser:Gilead Sciences: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Janssen: Honoraria. Fegan:Gilead Sciences: Honoraria; Roche: Honoraria; AbbVie: Honoraria. Yang:Gilead Sciences: Employment, Equity Ownership. Mitra:Gilead Sciences: Employment, Equity Ownership. Dyer:ONO Pharmaceuticals: Research Funding; Gilead Sciences: Consultancy, Other: Travel funding, Research Funding, Speakers Bureau; Roche: Consultancy, Speakers Bureau. Salles:Janssen: Consultancy, Honoraria; Gilead: Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Mundipharma: Honoraria; Roche/Genentech: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.