Abstract
Background
Suppression of uninvolved immunoglobulins is a common finding in multiple myeloma and the preservation of uninvolved immunoglobulins at diagnosis is associated with improved progression-free and overall survival. However, little is known about the impact of myeloma treatment on levels of uninvolved immunoglobulins, and the link between changes in uninvolved immunoglobulins during therapy and treatment response, disease progression and survival.
Methods
We identified patients who received therapy for newly diagnosed multiple myeloma at our institution between 2001 and 2014, and who had data available on absolute lymphocyte count (ALC) and quantitative uninvolved immunoglobulins (Ig) before commencing treatment. The ALC and levels of uninvolved Ig after 4 cycles of therapy were abstracted from the electronic medical record; patients who switched or stopped treatment, or died, before this time point were excluded. To assess change in ALC, the percentage difference in ALC between baseline and 4 cycles was calculated; for uninvolved Ig, the average of the percentage difference between baseline and 4 cycles for each uninvolved Ig (IgA and IgM for IgG myeloma, IgG and IgM for IgA myeloma, IgG and IgA for IgM and IgD myeloma, and IgG, IgM and IgA for light-chain only myeloma) was calculated. Treatment response at 4 cycles was retrospectively assigned according to International Myeloma Working Group criteria. Time to treatment failure (TTF) was defined as time from start of initial therapy to start of next line of therapy or death (if no additional treatment was received). A landmark analysis was used to calculate overall survival (OS) from the date of follow-up after 4 cycles of therapy. The Kruskal-Wallis, Chi-Square, and log rank tests were used to detect differences in medians, proportions, and survival times respectively.
Results
A total of 421 patients were included in this analysis. The median age was 63 years (range 33-91), 254 patients (60.3%) were male and median follow-up was 6.5 years (95% CI 5.6-7.3); the vast majority of patients had IgG (n=247 [58.7%]), IgA (n=98 [23.3%]) or light-chain only myeloma (n=68 [16.2%]). First line therapy comprised of pulse-dose dexamethasone (DEX, n=92 [21.9%]), lenalidomide-dexamethasone (RD, n=176 [41.8%]), bortezomib-dexamethasone (VD, n=22 [5.2%]), bortezomib-cyclophosphamide-dexamethasone (VCD, n=84 [20.0%]), and bortezomib-lenalidomide-dexamethasone (VRD, n=47 [11.2%]).
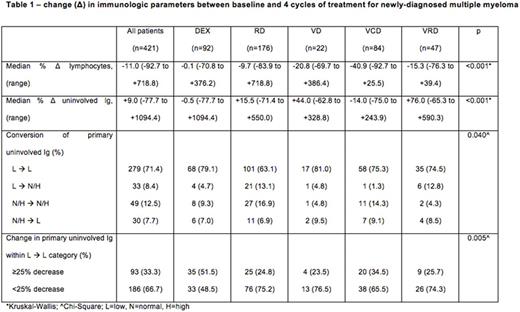
Across the entire cohort, the median change in ALC and uninvolved Ig after 4 cycles of treatment was -11.0% (range: -92.7 to +718.8) and +9.0% (-77.7 to +1094.4) respectively; treatment with VCD was associated with the greatest median declines in ALC (DEX: -0.1%; RD: -9.9%; VD: -20.8%; VCD: -40.9%; VRD: -15.3%) and uninvolved Ig (DEX: -0.5%; RD: +15.5%; VD: +44.0%; VCD: -14.0%; VRD: +76.0%, both p<0.001). Conversion from suppression to normalization of the primary uninvolved Ig (IgA in IgG myeloma, and IgG in all other myeloma types) after 4 cycles was seen more frequently with the use of RD (13.1%) and VRD (12.8%) compared to DEX (4.7%), VCD (1.3%), or VD (4.8%), χ2=21.8, p=0.040. When considering only patients in whom the primary uninvolved Ig remained suppressed between baseline and 4 cycles, a ≥25% reduction in the primary uninvolved Ig occurred more frequently with the use of DEX (51.5%) and VCD (34.5%) compared to RD (24.8%), VD (23.5%) or VRD (25.7%), χ2=15.1, p=0.005 (Table 1).
Although an average reduction in uninvolved Ig between baseline and 4 cycles (ΔIg<0) was independently associated with a lower likelihood of achieving very good partial response (VGPR) of better on multivariate analysis adjusting for age, sex and treatment regimen (OR=0.40 [0.24-0.63], p<0.001), there were no differences in TTF (2.0yrs vs. 2.0yrs, p=0.783) or OS (8.0yrs vs. 8.0yrs, p=0.721) between patients with ΔIg<0 (n=169) and those with ΔIg≥0 (n=222).
Conclusions
Myeloma treatments produce differential impacts on immune parameters, with VCD causing the greatest reduction in lymphocytes and uninvolved Ig, implying general targeting of plasma cells, in comparison to lenalidomide, which appeared to be more tumor-specific with relative sparing of lymphocytes and uninvolved Ig. While an average decrease in uninvolved Ig was an independent predictor of a lower likelihood of achieving VGPR or better after 4 cycles of therapy, it was not associated with a shorter TTF or poorer OS.
Kumar:BMS: Consultancy; Glycomimetics: Consultancy; Onyx: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Kesios: Consultancy; Millennium: Consultancy, Research Funding; Noxxon Pharma: Consultancy, Research Funding; Array BioPharma: Consultancy, Research Funding; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; AbbVie: Research Funding. Dispenzieri:GSK: Membership on an entity's Board of Directors or advisory committees; Prothena: Membership on an entity's Board of Directors or advisory committees; pfizer: Research Funding; Jannsen: Research Funding; Alnylam: Research Funding; Celgene: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kapoor:Amgen: Research Funding; Celgene: Research Funding; Takeda: Research Funding. Bergsagel:Amgen, BMS, Novartis, Incyte: Consultancy; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract