Abstract
Background
Despite significant progress, MM remains an incurable disease. Synergistic activity of immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) has been established in preclinical and clinical studies. Data with respect to combination of newer IMiDs and PIs are scant. Recently, a small study (n=32) found the novel therapeutic regimen consisting of carfilzomib, pomalidomide and dexamethasone (KPd) to be well-tolerated and active in RRMM patients, but only 7% of patients in that study had ECOG > 1. KPd has not been extensively studied outside of clinical trials. Therefore, we evaluated real-world outcomes of KPd in RRMM patients seen in our routine dysproteinemia practice.
Methods
Records of MM patients consecutively seen at Mayo Clinic, Rochester from 01/ 2013 to 05/ 2016 were reviewed. Forty-five MM patients who received KPD in the R/R setting were identified and included in the analysis. The international uniform response criteria (IMWG 2006) were used to assess response rates. Additionally, minor response (MR) was assessed to determine the clinical benefit rate (CBR or ≥MR). Toxicity was evaluated using the Common Terminology Criteria for Adverse Events version 4. Duration of response was calculated for patients who have achieved a PR or greater response from time of first response to progression of disease. The time-to-event analyses were performed from the time of initiation of KPd therapy using the Kaplan-Meier method. All but one patient received K at 20/27 mg/m2 (days 1, 2, 8, 9 15, 16) dosing, P at 2 mg or 4 mg (day 1-21), and dexamethasone at 20 or 40 mg (day 1, 8, 15, 22), with 28-day cycles.
Results
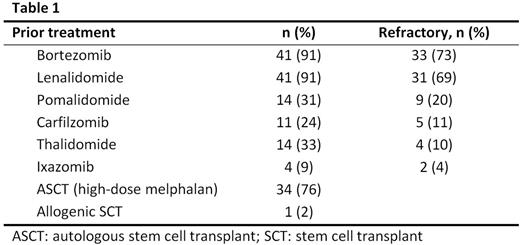
The median age at KPd initiation was 65 years (range 29-75), 25 (56%) patients were males. 71% had an ECOG performance score of 0 to 1, and 29% had an ECOG >1. Forty percent were mSMART standard risk, while 60% were intermediate to high risk. Median time from MM diagnosis to KPd was 44 months (range 6-188), and the median number of prior treatment lines was 4 (range 1-14). Three (7%) patients were quadruple refractory to bortezomib (V), lenalidomide (R), P, and K. Ten (23%) were triple refractory to V, R, and P or K. Twenty seven (60%) were dual refractory to V and R. Table 1 shows prior treatment agents and refractoriness. The median duration of treatment with KPd was 2 months (range 0.3-16.4). KPd was used as a bridge to autologous stem cell transplant (ASCT) in 5 patients. One patient discontinued therapy due to toxicity (infection, weakness).
Response was evaluable in 44 patients. The median time to first response was 1.4 months (range 0.5-6). Overall response rate (ORR) was 36%, [stringent complete response (sCR) in 1 (2%) patient, complete response (CR) in 3 (7%) patients, very good partial response (VGPR) in 2 (5%) patients, partial response (PR) in 10 (22%) patients]. Minimal response (MR) was seen in 3 (7%), leading to a CBR of 43%, while an additional 10 (23%) patients had stable disease (SD). Progressive disease (PD) was noted in 15 (34%) patients.
At the time of analysis 20 (43%) patients were deceased, all from PD. The median follow up from initiation of KPd was 16.3 months (95% CI: 8.6-19.4). The median overall survival (OS) from KPd was 16.1 months (95% CI: 10.6-20.4). Median OS for dual refractory was 15.6 (95% CI: 10.6-20.5). Median OS for triple/quadruple refractory was similar to those with lesser degree of refractoriness [19.1 (95% CI: 0.92 - 27.1) months vs 13.6 (95% CI: 8.9-20.5), p= 0.82]. The median progression-free survival (PFS) was 3.3 months (95% CI: 1.9-6.4) and the median time-to-next therapy (TTNT) was 3.5 months (95% CI: 2.2-6.7). The media duration of response was 12.9 months (95% CI: 2.5-19.6). The median OS from KPd for patients who achieved at least a PR was 20.4 months (95% CI: 13.6-NR), compared to 11 months (95% CI: 8.3-NR) for others (p=0.05).
Grade ≥3 hematologic toxicity was seen in 19 (42%) of evaluable patients [grade ≥3 neutropenia (29%), thrombocytopenia (31%) and anemia (22%)]. The most common non-hematologic adverse events were fatigue (38%), infections (31%), diarrhea (12%) and dyspnea (12%). Of the 4 (9%) patients who developed venous thromboembolism (VTE), two were on aspirin prophylaxis while the other two were not receiving any VTE prophylaxis.
Conclusions
KPd shows activity in RRMM patients although the short PFS reflects the challenges encountered with managing heavily pretreated and often less fit patients in the real world setting outside of clinical trials.
Dispenzieri:Prothena: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Jannsen: Research Funding; pfizer: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Alnylam: Research Funding. Kumar:BMS: Consultancy; Kesios: Consultancy; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Noxxon Pharma: Consultancy, Research Funding; Array BioPharma: Consultancy, Research Funding; Glycomimetics: Consultancy; AbbVie: Research Funding; Onyx: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Celgene: Consultancy, Research Funding. Kapoor:Amgen: Research Funding; Celgene: Research Funding; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.