Abstract
Anti-CD19 chimeric antigen receptor (CAR) T cell therapies for B cell malignancies have demonstrated the remarkable curative potential of T cell immunotherapies. However, in clinical trials anti-CD19-CAR T cells continue to trigger life threatening adverse events that are often associated with excessive cytokine release and excessive T-cell proliferation. We reasoned that the activation pathway of current CAR T cells could be altered to better regulate proliferation and cytokine secretion, and thus disentangle the correlation between cytokine release syndrome (CRS) and efficacy of T cell-based therapies.
Through protein engineering, we developed the ARTEMISTM (1) signaling platform which when expressed on primary T-cells results in a dramatic reduction of cytokine release during tumor cell lysis, without sacrificing efficacy. Using a human phage display library, we also identified several human CD19 antibodies with improved specificity and affinity that will be less immunogenic as compared to the murine-derived anti-CD19 antibodies that are currently used in most trials. Our lead antibody clone CD19(7) was then engineered into both CD28z-CAR and ARTEMISTM platforms for comparison.
When tested in vitro, both CD19(7)-ARTEMISTM T cells and CD19(7)-CD28z-CAR T cells specifically lysed multiple CD19+ leukemia and lymphoma cell lines with similar potencies. However, during the 16 hour killing assays, ARTEMIS™ T cells secreted over 1000-fold less IL-2 and dramatically lower levels of IFN-γ, GM-CSF, IL-10 and IL-6. ARTEMISTM T cells also accumulated less PD-1, LAG3, and TIM3 on their surface during culturing and following in vitro killing, indicating a diminished propensity for exhaustion. Furthermore, during in vitro T cell expansion, ARTEMISTM cells were enriched for naïve/central memory subpopulations, had lower expression of granzyme B, a marker of terminal differentiation, and had reduced rates of receptor internalization upon antigen engagement. These characteristics suggest that T-cells activated through the ARTEMISTM receptor will have improved persistence and long-term proliferation potential, as well as a safer, more controlled cytokine release when used for T-cell therapies.
When tested in vivo against CD19+ Raji systematic lymphoma xenografts, intravenous administration of CD19(7)-ARTEMISTM T cells caused rapid, complete, and lasting tumor regression that was better than that achieved with an equal dose of CD19(7)-CD28z-CAR T cells (Figure 1). In agreement with our in vitro data, mice treated with ARTEMISTM T cells had nearly undetectable levels of cytokines in their blood at 24 hours post dosing, a time in which CD19(7)-CAR-treated mice had markedly elevated levels of human IFN-γ, IL-2, TNFα, and IL-10.
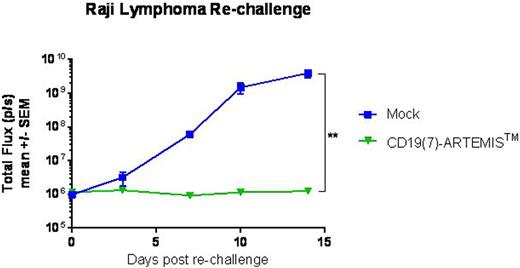
While flow cytometry analysis of the peripheral blood showed that CD19(7)-CAR T cells expanded more rapidly in mice, CD19(7)-ARTEMISTM T cells better controlled Raji tumor growth and were negative for PD-1 expression which was high on circulating CAR T cells. At 7 weeks post dosing, a time when all ARTEMISTM T cell-treated mice had no detectable tumors, they were re-challenged with Raji lymphoma. While tumors grew rapidly in control mice, ARTEMISTM T cell-treated mice resisted the Raji lymphoma re-challenge, indicating that ARTEMISTM T cells persisted in these mice despite the absence of tumors and remained antigen-responsive (Figure 2).
Our data demonstrates that CD19(7)-ARTEMISTM T cells are highly potent against lymphoma preclinical models while releasing drastically lower levels of cytokines. Thus we have developed and pre-clinically validated a novel fully human anti-CD19 T cell therapy that has the potential to persist longer in patients and, importantly, presents a lower risk of cytokine-related toxicities without compromising efficacy. A clinical trial testing CD19(7)-ARTEMISTM T cell therapy in humans is expected to begin in 2017.
Raji lymphoma tumor growth in NSG mice treated with either donor-matched untransduced T cells (Mock), CD19(7)-CAR, or CD19(7)-ARTEMISTM T cells (5x106 receptor-positive cells per mouse)
Raji lymphoma tumor growth in NSG mice treated with either donor-matched untransduced T cells (Mock), CD19(7)-CAR, or CD19(7)-ARTEMISTM T cells (5x106 receptor-positive cells per mouse)
Raji lymphoma tumor growth in NSG mice previously treated with CD19(7)-ARTEMISTM T cells who had complete regression (0.5x106 Raji cells/mouse). As controls, Raji-naïve mice were implanted with Raji cells following an injection of Mock T cells.
(1)ARTEMISTM is trademarked by Eureka Therapeutics, Inc.
Raji lymphoma tumor growth in NSG mice previously treated with CD19(7)-ARTEMISTM T cells who had complete regression (0.5x106 Raji cells/mouse). As controls, Raji-naïve mice were implanted with Raji cells following an injection of Mock T cells.
(1)ARTEMISTM is trademarked by Eureka Therapeutics, Inc.
Liu:Eureka Therapeutics: Employment, Equity Ownership, Patents & Royalties. Long:Eureka Therapeutics: Employment, Equity Ownership. Green:Eureka Therapeutics: Employment. Horan:Eureka Therapeutics: Employment. Zimdahl:Eureka Therapeutics: Employment. Liu:Eureka Therapeutics: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.