Abstract
Background: Follicular lymphoma (FL) is a highly heterogeneous disease. Patients with early progression of disease (POD) have a remarkably poor outcome. Approximately 20% of patients with symptomatic, advanced stage FL experience POD within 24 months of first-line treatment (POD24) and have a 5-year overall survival (OS) of <50%, compared to >85% for patients without POD24 (Casulo et al, JCO 2015; Jurinovic et al, Blood 2016). The optimal salvage treatment for these high-risk patients is unclear. We aimed to assess the role of autologous stem cell transplantation (ASCT) applied as 2nd-line treatment for patients with POD24 who -in principle- qualified for dose-intensified regimens.
Methods: Patients with advanced stage FL from 2 successive randomized trials of the German Low Grade Lymphoma Study Group (GLSG1996 [MCP versus CHOP] and GLSG2000 [CHOP versus R-CHOP], followed by randomization for ASCT consolidation versus interferon maintenance, respectively) were eligible for retrospective analysis of 2nd-line treatment if they had progressive, relapsed or refractory FL (ie, less than a partial response), were <65 years, required treatment according to our established criteria, and had not received prior ASCT. Three patients who received an allogeneic transplant (alloTx) as 2nd-line treatment were excluded from this analysis. POD24 was defined as progressive, relapsed or refractory disease within 24 months after 1st-line treatment. Progression-free survival (PFS) and OS were calculated from initiation of 2nd-line treatment (2nd-line PFS and 2nd line-OS, respectively).
Results: The evaluable 162 patients were enriched for POD24 (113/162 [70%]). POD24 patients were more likely to receive ASCT as 2nd-line treatment (52/113 [46%] versus 11/49 [22%], p=0.008), whereas rituximab was more commonly added to 2nd-line regimens in patients without POD24 (48% versus 69%, p=0.018). With a median follow-up of 9.4 years, POD24 patients had a significantly shorter 5-year 2nd-line PFS and OS with 35% versus 53% (p=0.04) and 60% versus 83% (p=0.02), respectively, compared to patients without POD24.
Within the POD24 group, 52 patients (46%) received ASCT, 54 (48%) received rituximab-containing regimens, 25 (22%) received both ASCT and rituximab as part of their 2nd-line treatment. Patients in the ASCT arm were younger (median age 47 versus 51 years, p=0.018) and more frequently male (73% versus 51%, p=0.026). The distribution of high-risk FLIPI (39% versus 46%, p=0.60), ECOG performance status >1 (10% versus 21%, p=0.15) and the addition of rituximab (48% versus 48%, p=1.0) was not significantly different between the ASCT and no-ASCT groups.
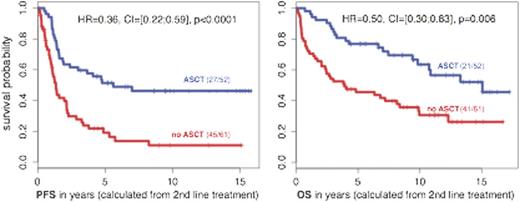
In univariate analysis, ASCT for POD24 patients was associated with significantly higher 5-year 2nd-line PFS and OS rates of 51% versus 19% and 77% versus 46% (CI=[35;60]), respectively (Figure). The hazard ratios (HR) for 2nd-line PFS and OS were 0.36 (CI=[0.22;0.59], p<0.0001) and 0.50 (CI=[0.30;0.83], p=0.006).
Multivariate analysis demonstrated that ASCT had a stronger impact on favorable treatment outcome compared to the addition of rituximab: the HRs of ASCT for 2nd-line PFS and 2nd-line OS were 0.37 (CI=[0.21;0.63], p=0.0003) and 0.34 (CI=[0.21;0.56], p<0.0001), whereas the HRs of rituximab were 0.63 (CI=[0.39;1.00], p=0.05) and 0.64 (CI=[0.39;1.08], p=0.09), respectively.
Finally, to account for the selection bias associated with retrospective studies, we considered patients as ASCT if there had been an attempt to collect an autograft. This included an additional 13 patients from the no-ASCT group and another patient who ultimately received an alloTx as 2nd-line treatment. In this intention-to-treat-like analysis the benefit of ASCT remained statistically significant with a HR=0.48 for 2nd line PFS (CI=[0.30;0.77], p=0.002) and a HR=0.50 for 2nd-line OS (CI=[0.30;0.83], p=0.006).
Conclusion: This retrospective analysis suggests that ASCT is an effective 2nd-line treatment option for patients with high-risk FL as defined by the early progression of disease (POD24) who qualify for dose-intensified protocols, and should be evaluated in prospective clinical trials. It remains to be determined if ASCT is also an effective 1st-line treatment strategy for selected patients identified to be high-risk by pre-treatment risk classifiers, such as the recently established clinicogenetic risk-model m7-FLIPI.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.