Abstract
Background: Pain in sickle cell disease (SCD) has been thought to be episodic, but more recent evidence has shown that individuals in this population also suffer from chronic pain likely resulting from central or peripheral neural damage (neuropathic pain). There is accumulating evidence from human and animal studies indicating potential neuropathic pain in SCD. A number of valid and reliable measures of neuropathic pain have been used to differentiate neuropathic from non-neuropathic types of pain. PAINReportIt, which takes about 10 to 18 minutes to complete, is a computer based self-report pain assessment tool based on the 1970 version of the McGill Pain Questionnaire. From PAINReportIt, a new subscale has been proposed as a measure of neuropathic pain that sums the number of neuropathic pain quality words selected. The PAINReportIt number of neuropathic pain (PR-NNP) scale, however, lacks validation in patients with SCD.
Aim: The purpose of this study was to determine the construct validity for the PR-NNP by examining the associations between the PR-NNP and other valid and reliable measures of neuropathic pain (self-administered Leeds Assessment of Neuropathic Symptoms and Signs [S-LANSS] and the Neuropathic Pain Symptom Inventory [NPSI]) among adults with SCD. We hypothesized that the PR-NNP scores would be significantly correlated with S-LANSS and NPSI scores.
Methods: This prospective instrument validation study was conducted in an ambulatory research setting with 79 adults diagnosed with SCD who had chronic pain within the prior 12 months (>3 on a 0-10 pain scale). The sample mean age was 36.0 ± 11.5 [ranged from 19-74 years], 63% were female, and 97% reported they were African American. The participants were asked to complete self-reported pain measures (PR-NNP, S-LANSS, NPSI, and PR-NNoc [number of nociceptive pain words]). Descriptive, correlational, and regression analyses were used.
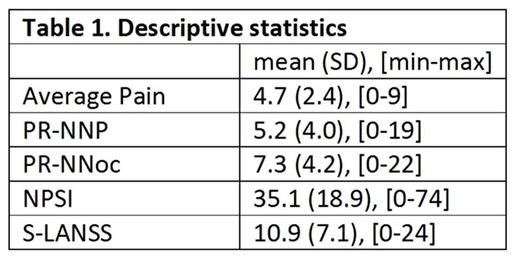
Results: Mean scores for average pain intensity, PR-NNP, NSPI, S-LANSS, and PR-NNoc appear in Table 1.
Bivariate results indicated moderate correlation between the two validated measures of neuropathic pain (NPSI and S-LANSS; r= .57, p=.000). The NPSI was moderately correlated with PR-NNP (r= .43, p=.000), and weakly correlated with PR-NNoc (r=.35, p=.002). For S-LANSS, there was a moderate correlation with PR-NNP (r=0.41, p=.000) and a weak correlation with PR-NNoc (r=.30, p=.007). There was a weak correlation between average pain intensity and NPSI and S-LANSS, r=.37, p=.001 and r=.36, p=.001, respectively. Regression analysis including average pain intensity, PR-NNP, and PR-NNoc as predictors showed that controlling for PR-NNP and average pain, PR-NNoc was not significantly associated with either NPSI (p=.930) or S-LANSS (p=.731), while each point of increase in PR-NNP was associated with an increase of 1.9 (p=.004) in NPSI and of 0.8 (p=.003) in S-LANSS. The same analysis showed that a one point increase in the average pain intensity was associated with an increase of 2.7 (p=.001) in NPSI and of 1.0 (p=.001) in S-LANSS.
Conclusions: Both average pain intensity and PR-NNP but not PR-NNoc have unique explanatory properties of both indicators of neuropathic pain (NPSI and S-LANSS). These findings support the construct validity of the PR-NNP as a potential screening tool for neuropathic pain in patients with SCD. Validation of PR-NNP is important for future neuropathic pain research in the sickle cell population, particularly in cases of multi-site trials, and in cases where the practitioner can detect the potential presence of neuropathic pain without use of expensive equipment. These findings are important because pain management in the sickle cell population often includes opioids, but easy and early detection of neuropathic pain could result in an opioid sparing pain management approach in this population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.