Abstract
Introduction
The treatment landscape for many mature B-cell malignancies is evolving rapidly, with patients and clinicians facing increasingly complex choices about therapeutic options that differ in efficacy, toxicity and cost. Accounting for around a quarter of all haematological cancer diagnoses, multiple myeloma (MM) and chronic lymphocytic leukaemia (CLL) are two conditions where increases in the number and combinations of potentially life-prolonging therapies has been particularly marked; ranging from the use of single alkylating agents to immunomodulatory drugs and proteasome inhibitors for MM, and combination chemotherapy, immuno-chemotherapy, novel monoclonal antibodies and tyrosine-kinase inhibitors (TKIs) for CLL. Contemporary data enabling the success of such therapeutic changes to be evaluated in the general patient population is, however, lacking. With centralized diagnostics and a unified clinical network covering a catchment population of 4 million, the UK's Haematological Malignancy Research Network (www.hmrn.org) was specifically established to provide timely real-world data to answer such questions; and findings from this unique population-based cohort are reported here.
Methods
Patients newly diagnosed 2004-13 with MM (n=2084) or CLL (n=1866) were followed-up until January 2016. Demographic, prognostic, first-line treatment and outcome data for the time-periods 2004-07, 2008-10 and 2011-15 were examined using standard statistical methods; relative survival (RS) was estimated using national life tables.
Results
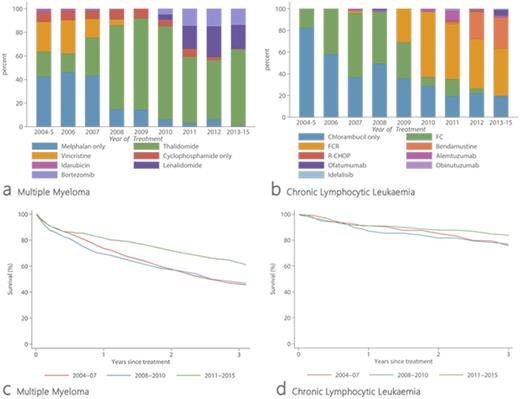
The median age at diagnosis of MM was 73 years (17% <60 years); 39% of patients presented with an ISS score of III and 25% were asymptomatic (CRAB score 0). In total, 1514 (73%) patients received first-line chemotherapy either at diagnosis or as a consequence of disease progression. Regimens were classified by their main agent, and the therapy changes over the 11-year period are shown in Figure 1a; in 2004-07, 44% of treated patients received single-agent alkylating therapy, in 2008-10 76% were treated with combination immunomodulatory therapy and by 2011-15 this had increased to 92%. The 3-year overall survival (OS) and RS estimates for all patients combined were 45.9% (95% Confidence Interval 43.4-48.4) and 52.0% (49.1-54.8) respectively. Differences in outcome by treatment year are clearly evident (Figure 1b): 3-year RS 2004-07, 46.5% (41.8-51.2); 2008-10, 48.4% (43.5-53.2); and 2011-15, 62.1% (56.8-66.9). The improvement in survival for patients treated in 2011-15 compared to 2004-07 was confirmed by multivariate Cox regression (Hazard Ratio 0.65, 0.56-0.76).
With a median diagnostic age of 71 years (18% <60 years); the majority of CLL patients had early-stage disease (BinetStage A, 78%). In total 547 patients were treated with first-line chemotherapy, with the regimen again changing over time (Figure 1c). Patients treated 2004-07 generally received single alkylating agents (56%) or combination chemotherapy (42%), by 2008-10 32% of patients had a monoclonal antibody added to chemotherapy (chemo-immunotherapy), increasing to 72% among those treated 2011-15. The 3-year OS and RS for all treated patients combined were 69.5% (65.3-73.3) and 80.3% (75.5-84.3) respectively. However, there was no incremental statistically significant change in 3-year RS (Figure 1d); 2004-07, 76.4% (65.2-84.4); 2008-10, 78.3% (69.8-84.6); and 2011-15 84% (76.3-89.4); and taking 2004-07 as the reference, the corresponding hazard ratios for the 3 time-periods were 1 (reference), 1.00 (0.78-1.37) and 0.79 (0.58-1.09).
The cost implications of the changing treatment landscape are currently being examined, and by December 2016 the findings presented above will include more recently diagnosed patients (2014-15), which is particularly pertinent for CLL, where a step-change may have occurred due to the introduction of TKIs.
Conclusions
Our analyses confirm that first-line chemotherapy for MM and CLL is changing markedly; highlighting the importance of monitoring the impact of therapeutic change in a real-world setting. The improvement in MM survival currently contrasts with CLL, suggesting that encouraging results from clinical trials may not always translate directly into similar improvements at a population level. Clearly, additional analysis of data from patients diagnosed >2014 are required.
Smith:Novartis: Research Funding; Janssen-Cilag: Research Funding; Amgen: Research Funding; Celgene: Research Funding. Cook:Celgene: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Takeda Oncology: Consultancy, Research Funding, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Glycomimetics: Consultancy. Patmore:Roche: Honoraria; Janssen Cilag: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.