Abstract
MYC expression is frequently dysregulated in multiple myeloma (MM). In one comprehensive study, MYC structural variations (SV) were found in nearly half of MM cases (Affer et al. Leukemia 2014). The prevalence was higher in hyperdiploid (HRD) tumors (65%) compared to non-hyperdiploid (NHRD) tumors (36%). The large amount of tumor DNA required for all of the genomic studies performed may have biased the samples analyzed (e.g., to those with higher tumor burdens). To validate the findings of recurrent MYC SV in another dataset, we analyzed the CoMMpass data. We analyzed long-insert whole genome sequencing (WGS) data from diagnostic samples in 420 patients from the IA7 release (dbGAP phs000748) for SV. The results of clinical data, processed WES (SNV), RNASeq (gene expression) and WGS (copy number) data from IA8 (http://research.themmrf.org) were used to calculate survival, RAS and NFKB pathway mutation (WES), TC class (RNA), NFKB index (RNA) and hyperdiploid index (WGS).
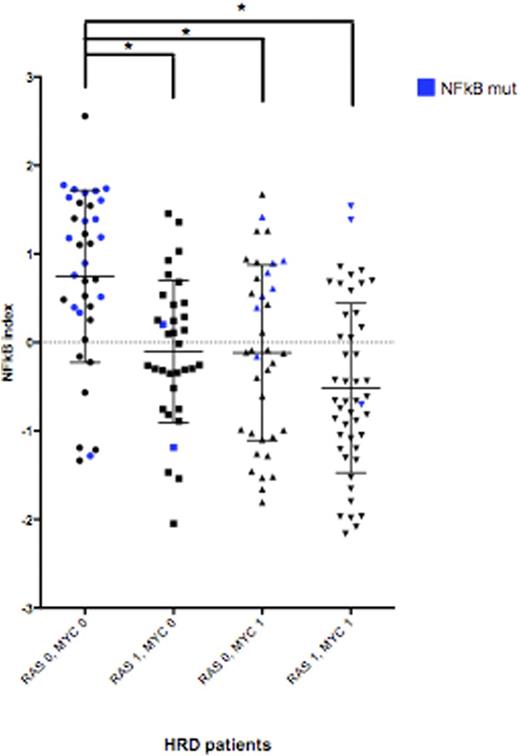
MYC SVs were identified in 38% of tumors. They were present in 53% HRD and 28% NHRD, and by TC in 55% D1, 43% D2, 36% MMSET, 26% MAF, 13% CCND. Juxtaposition of an Ig enhancer (IgH, IgK, IgL) close to MYC was the most common MYC SV, representing ~40% of the MYC SV. Other enhancers identified have mostly been reported previously, with the most frequent being NSMCE2 (12% of SV) and TXNDC5 (5% of SV). Intrachromosomal SV (deletions, inversions, duplications) not associated with any known enhancer were also frequent (18% of SV). As expected, MYC expression was higher in tumors with MYC SV compared to those without (~2.4 fold, p-value<0.0001). We used Gene Set Enrichment Analysis (GSEA) to identify activated pathways that might substitute for MYC in HRD patients without MYC SV and observed significant activation of the NFkB pathway. Interestingly, examining patients with RAS/MAPK pathway (NRAS, KRAS, BRAF, FGFR3 - abbreviated RAS) mutations (identified in 50% of all patients) the same pattern was observed: in the absence of RAS mutation, there was a significantly higher NFkB index. In general, the HRD tumors seem to have either activation of RAS and/or MYC, or activation of NFkB (Figure). There was no difference in overall survival in patients with versus those without MYC SV.
We developed a clinically applicable sequencing platform to identify MYC SV, which cannot be reliably identified by FISH. We sought to validate this targeted capture approach, where in addition to the coding exons of 81 interesting genes described previously (M3P), we also pulled down the region surrounding MYC (2 Mb), IgH (0.5 Mb), IgK (50 kb) and IgL (100 kb) allowing us to additionally identify SV in MYC and Ig loci. Using this approach we identified IgH translocations in 29/30 samples with translocations previously identified by FISH (97%). Moreover, we identified MYC SV in 19/22 patients with SV previously identified by mate-pair WGS (86%). Importantly, sequencing identified the precise translocation breakpoint, and identity of the enhancer dysregulating MYC, which may be important variables. In one informative patient two different MYC SV were present at diagnosis, only one of which was still present following a partial response to four cycles of lenalidomide and dexamethasone. This suggests that the two MYC SV are in different subclones, one of which was much more sensitive to the treatment. Interestingly, the enhancer dysregulating MYC in the sensitive subclone harbors 5 strong Ikaros binding sites identified by ChIPseq, suggesting one intriguing mechanism for sensitivity to lenalidomide.
To summarize, we verified in a large dataset that MYC expression is frequently dysregulated by SV in MM (38%), and the RAS/MAPK (50%) and NFKB (23%) pathways are frequently activated by mutations. Surprisingly, given their generally good prognosis, nearly half of HRD tumors seem to be MYC driven, while this is true for only a quarter of NHRD tumors. HRD tumors not driven by MYC or RAS appear to be driven by NFKB. Remarkably, the pathways most commonly activated by mutation in MM: CCND, MYC, RAS, NFKB are common to many cancers and have been studied extensively individually. To understand their clinical impact in MM we have developed a comprehensive custom capture sequencing panel that identified 97% of IgH translocations, 86% of MYC SV, and as well as SNV and CNV of 81 recurrently mutated genes. It will be important to include such a comprehensive genetic analysis to complement clinical trials in the future.
Stewart:Bristol Myers Squibb: Consultancy; Celgene: Consultancy; Takeda Oncology: Consultancy; Janssen Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.