Abstract
Background
Non-cancerous medical conditions have a profound impact on the health of patients with malignancies. In individuals with lung cancer, the likelihood of death due to other causes is higher than that of the general population. It is therefore essential that patients with hematologic/oncologic diseases be effectively represented in non-oncologic clinical trials. Despite that truism, patients with cancer or with hematological impairments are typically excluded from clinical trials due to safety concerns. The frequency and degree to which hematological parameters or a diagnosis of cancer serve to exclude patients from practice-changing clinical studies in cardiology have yet to be determined. Whether studies that exclude patients based on such parameters have a lower incidence rate of major bleeding than those that do not is also unknown.
Methods
We focused on acute coronary syndrome (ACS). An investigation of the hematologic/oncologic exclusion profile of landmark studies of ACS was conducted. We searched The New England Journal of Medicine for all original research studies of ACS published between 2006 and 2016. Hematologic/oncologic exclusion criteria were reviewed. The nature of those parameters (laboratory value, disease state, medication use, clinical risk assessment) was analysed. The number of criteria per study as well as the percentage of participants excluded for meeting those criteria were recorded. The incidence of major bleeding (defined by TIMI bleeding criteria) among patients randomised into the interventional arms was compared between studies that excluded patients with particular derangements and those that did not.
Results
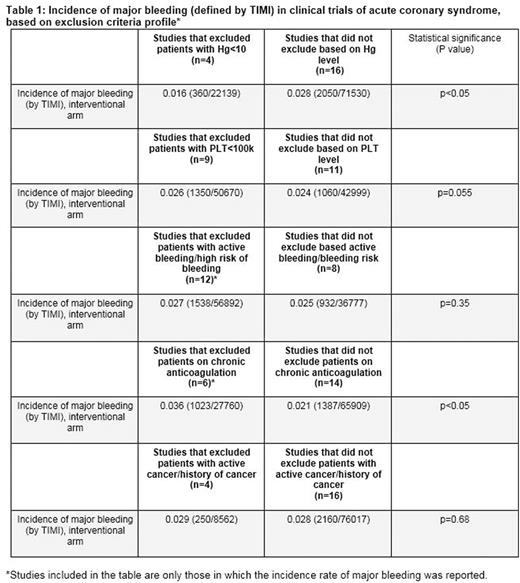
Thirty randomised clinical trials were found with a total number of patients of 235,981. Two thirds (20 out of 30) of the studies had at least one hematologic/oncologic exclusion criteria. The mean number of hematologic/oncologic exclusion criteria per study was 1.7. The most common exclusion parameter was laboratory-based. Ten studies (33.3%) specified cell count value as a cutoff for participation. Haemoglobin threshold (<10.0 mg/dl) was included in 4 studies (13.3%), while a platelet cutoff (<100k, >700k) was chosen in 9 publications (30%). Twelve studies (40%) issued active bleeding diathesis while six (20%) included "high-risk" for bleeding as exclusion criteria. Eight studies (26.6%) excluded patients who were chronically on oral anticoagulation. Six studies (20%) excluded patients with history of cancer. Seven studies (23.3%) had at least one subjective parameter in the list of exclusion criteria, such as "concerning lab abnormality", "high-risk" for bleeding, or "severe haematological disease". The aggregate incidence rate of major bleeding was significantly higher in studies that did not exclude for haemoglobin levels lower than 10 mg/dl compared with those that did (2.8% versus 1.6%, p value<0.05). Major bleeding rates were higher in studies that excluded patients on chronic anticoagulation than in studies that did not (3.6% versus 2.1%, p value<0.05). There was no significant difference in the incidence of major bleeding between studies that excluded patients with thrombocytopenia (platelets<100k), active bleeding or history of malignancy and studies that did not (Table 1). Not a single publication reported the fraction of patients excluded due to hematological impairments or history of cancer.
Conclusions
Hematological parameters as well as a medical history of a cancer commonly serve as exclusion criteria in studies of patients with ACS. Our analysis suggests that there may be a need for better standardization of exclusion criteria in studies in cardiovascular medicine, given that almost a quarter of high-quality studies of ACS include at least one inherently subjective parameter. The results provide support for uniformly setting severe anemia as exclusion criteria in studies of ACS until further data is available. Interestingly, enrolling patients with thrombocytopenia (platelets<100k), active bleeding or history of malignancy translated into similar rates of major bleeding, suggesting that some patient populations may be unnecessarily restricted from participating in clinical trials. Lastly, data pertaining to the subgroups of patients who were excluded based on those impairments is lacking and should be reported in future studies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.