Abstract
Background: Transfusion is a standard therapy for acute and chronic complications of sickle cell disease (SCD). They are complicated by a high prevalence of red cell antigen alloimmunization, which is not seen in other frequently transfused patients. Reasons for this increased prevalence are unclear. Some studies have suggested genetic predisposition to alloimmunization in all transfused patient populations. Others suggested that mismatched red cell antigens occurring commonly between blood donors of European ancestry and patients with SCD who are of African ancestry is a primary cause in SCD. Accordingly, phenotypic antigen matching and transfusion from African American donors are proposed strategies to minimize alloimmunization risk. We hypothesized that if these population differences are the etiologic basis that analysis of ancestry at the genetic level could identify significant genetic susceptibility contributions and localize susceptibility loci.
Methods: The Bethesda Sickle Cell Cohort Study consisting of adults evaluated at NIH from 2001-2015 (n=721) was reviewed for transfusion history and the presence of allo-antibodies confirmed with a blood bank. Genomic DNA was genotyped with an admixture panel consisting of 2251 ancestry informative SNPs in 359 patients and in HapMap controls. A final panel of 1622 SNPs was used to infer ancestry proportions using Structure assuming 3 ancestral populations. Ancestry was analyzed in regression models along with clinical variables. Genome wide admixture mapping was performed to identify regions of the genome where the ancestry proportions differed significantly between cases and controls. We used AncestryMap for a log-additive case control study assuming various risk models.
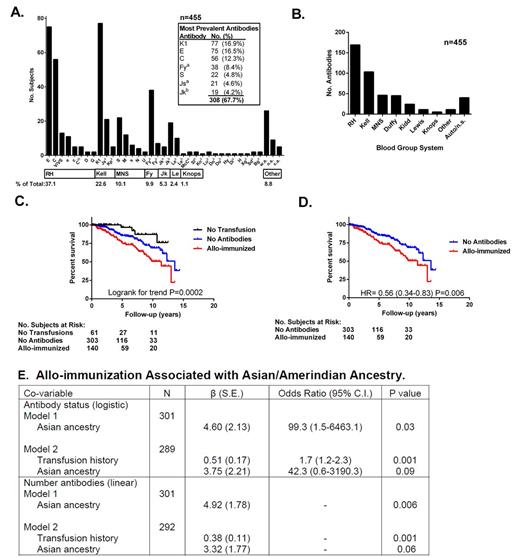
Results: Five hundred fifty four subjects (77% of cohort) had a transfusion history with 153 (27.6 %) with allo-antibodies, 336 (60.6 %) with a history of transfusions and no antibodies and 65 (11.7 %) with no prior transfusions. Sixty five percent had a history of more than 1 antibody. The RH, Kell, MNS and Duffy systems were most commonly associated with antibodies (Fig 1A and B). Clinical factors associated with cases in univariate analysis included transfusion burden (P<0.0001), pain hospitalizations (P=0.0007), hemoglobin (P=0.02), ferritin (P=0.002), alkaline phosphatase (P=0.02) and ESR (P=0.05). Only transfusion history remained significant in multivariable models, both when using logistic (antibody status, β=0.56, P=6.31 x 10-6) or linear (number of antibodies, β=0.35, P=0.0004) regressions. Given the univariate associations with pain hospitalizations and ferritin which are known mortality risk factors, it was not surprising that allo-immunization was associated with mortality when compared to the no antibody and non-transfusion groups (Fig 1C; median follow-up 3.8 years, range 0-14.4). The absence of allo-immunization showed decreased risk of death when limited to only those with prior transfusions (Fig 1D; Hazard Ratio 0.53, P=0.006). When considering genotype data, Asian/Amerindian ancestry was associated with antibody status (logistic, β=4.60, P=0.03) and number of antibodies (linear, β=4.92, P=0.006) by regression, while African and European ancestry were not. The relative ancestry contribution remained unchanged when the number of prior transfusions was also included in both models, P=0.09 and P=0.06, respectively (Fig 1E). Genetic data also indicated that 21% (n=77) of genotyped subjects were not admixed (100% African ancestry) which violates AncestryMap's assumption that all subjects are admixed. These subjects were removed from the admixture scan: an analysis of 81 cases and 159 controls with prior transfusions is underway to identify specific susceptibility loci.
Conclusions: In a large cohort of adults with SCD, the prevalence of red cell alloimmunization is 27.6%, with approximately 60% of antibodies attributable to RH and Kell. Asian/Amerindian ancestry is a risk factor, as is the number of prior transfusions. This study demonstrates genetic susceptibility to this complication which is associated with an ancestral population not typically considered in SCD. Identification of additional Amerindian ancestry SNPs will help to fine map susceptibility loci. Long term studies will be necessary to determine the relative contribution of alloimmunization to mortality in adults with SCD.
Kato:Mast Therapeutics: Consultancy; Bayer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract