Abstract
Background: Children with ITP for ≥6 months who completed a romiplostim phase 1/2 or phase 3 parent study could enroll in an open-label long-term extension study, data from which is presented here.
Methods: Patients enrolled at 28 sites in the US, Canada, Spain, and Australia. All patients received SC romiplostim once weekly. The initial dose was the final dose from the parent study or 1 µg/kg for patients previously receiving placebo, adjusted from 1−10 µg/kg to target platelet counts of 50−200×109/L. Incidence of adverse events (AEs) was the primary endpoint.
Results: As of 24 Feb 2016, 66 patients entered the extension study; 65 received romiplostim for up to 6.2 years. At baseline, median (min-max) age was 11 (3-18) years; 56% were female; 61% were white, 14% African American, 14% Hispanic/Latino, 9% Asian, and 3% other; 9.1% had prior splenectomy. Median (min-max) baseline platelet count was 27.5 (2-458)×109/L.
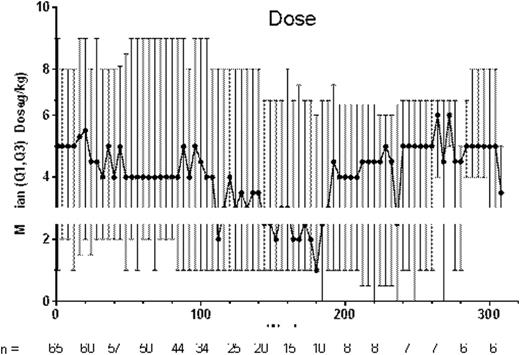
Median (min-max) treatment duration was 100 (5-321) weeks. Median (min-max) average weekly romiplostim dose was 4.8 (0.1-10.0) µg/kg, which included escalation to a stable dose; 19 patients started on 1 μg/kg. After ~week 200 (n ≤8 patients), the median dose was observed to fluctuate. All 65 patients received their doses per protocol >90% of the time; 18 patients missed ≥1 dose due to noncompliance for a total of 41 times. Reasons for discontinuing treatment (n = 22, 33%) included consent withdrawn (n = 8), required other therapy (n = 4), noncompliance (n = 3), administrative decision (n = 3), treatment no longer needed (n = 1), per protocol (n = 1), and AE (n = 2) (asthenia, headache, dehydration, and vomiting in one patient and anxiety in the other, per investigator, none of the AEs were treatment-related); 43 (65%) patients continued in the study. Fifty-two serious AEs occurred in 17 patients, 3 deemed treatment-related (anemia, epistaxis, and thrombocytopenia). Bleeding AEs occurred in 56 patients; 5 deemed treatment-related (gingival bleeding, petechiae, injection site bruising, injection site hematoma, and epistaxis). Bleeding AEs occurring in ≥10 patients included contusion (n = 30), epistaxis (n = 29), petechiae (n = 19), and gingival bleeding (n = 12). No thrombotic events were reported. There were no peripheral blood abnormalities to warrant a bone marrow examination. After sporadic platelet responses and negative antibody (Ab) results in the parent study, a patient left the extension due to a need for other therapies and was then identified to have anti-romiplostim neutralizing Ab which were not present on retesting 3 and 6 months later. No patients had anti-TPO neutralizing Ab.
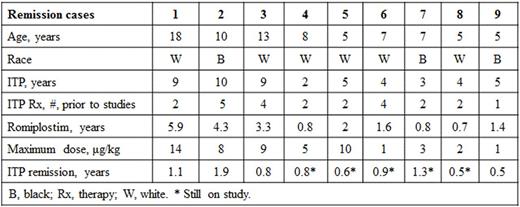
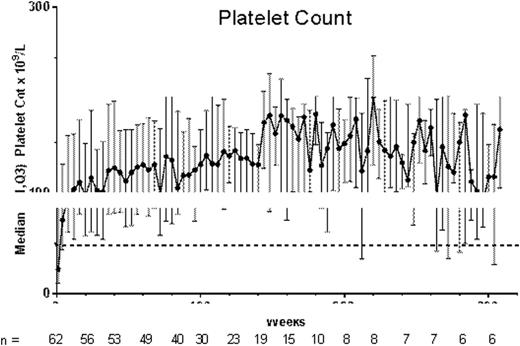
From week 2 on, median platelet counts remained >50×109/L; platelet counts were >100×109/L at most timepoints, despite an observed decrease in the median dose from 4-5 μg/kg to 2-3 μg/kg around week 160 (Figure). For 15 patients (23%), the first study week was the first week receiving romiplostim (previously these patients received placebo). Nearly all (94%, 61/65) patients had a platelet response (median platelet counts for a month ≥50×109/L); the mean (SD) % of months with a platelet response was 77% (33%). Most (72%, 47/65) patients had a platelet response ≥75% of the time and over half (58%, 38/65) of patients had a platelet response ≥90% of the time. Nine (14%) patients entered remission (Table), defined here as platelet counts ≥50×109/L for 24 weeks with no ITP treatments; these patients, 5 boys and 4 girls, none with prior splenectomy, had ITP for a median (min-max) of 5 (2-10) years and had received romiplostim for a median (min-max) of 1.6 (0.7-6.2) years. Fifty-eight (89%) patients (or caregivers) self-administered romiplostim. Twenty-three (35%) patients received rescue medications.
Conclusion: Over 6 years of data from this ongoing open-label extension study of romiplostim in children with ITP show that >90% of children achieved a platelet response with romiplostim, most responding ≥75% of the time. The safety profile was overall tolerable, similar to that in past studies. Some children (9/66) with longstanding ITP entered remission after receiving romiplostim.
Bussel:Symphogen: Membership on an entity's Board of Directors or advisory committees; Physicians Education Resource: Speakers Bureau; Immunomedics: Research Funding; Cangene: Research Funding; UpToDate: Patents & Royalties; Boehringer Ingelheim: Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Protalex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prophylix Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Momenta Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Research Funding; Sysmex: Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; BiologicTx: Research Funding; Rigel Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Tarantino:Biogen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Grifols: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Baxalta: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees. Blanchette:Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer Healthcare: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Baxter: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Data safety monitoring boards , Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Octapharma: Other: Data safety monitoring boards . Wang:Amgen Inc.: Employment, Equity Ownership. Mehta:Amgen Inc.: Employment, Equity Ownership. Eisen:Amgen Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.