Abstract
Background:
EXTEND (June 2006 to July 2015) was an open-label, dose-adjustment extension study to evaluate the safety and efficacy of eltrombopag for the treatment of patients with chronic immune thrombocytopenia purpura (cITP) who had previously been enrolled in a pivotal eltrombopag trial. CITP may lead to fatigue and interference with daily activities, and affect patient functioning and experience i.e., health-related quality of life (HRQoL).
Objective:
To investigate the association between platelet response and HRQoL reported by patients in the EXTEND study, employing widely used and well-validated measures.
Methods:
Four standard HRQoL instruments were used in this study: SF-36v2 including Physical Component Summary (PCS) and Mental Component Summary (MCS) to measure general physical and mental health status; Motivation and Energy Inventory Short Form (MEI-SF) to measure motivation and energy; Fatigue Subscale of FACIT (FACIT-F) to measure symptoms of fatigue; and FACT-Thrombocytopenia Subscale Six-Item Extract (FACT-Th6) to measure concerns related to bleeding and bruising and their impact on usual activities. The four instruments were used at baseline (BL) and at a frequency of every 3 months until last on-treatment assessment. Patients were blinded to their current platelet count results before completing the questionnaires. HRQoL scores and mean platelet count based on clinical assessments were calculated during time periods post-baseline (BL) to <3 months, ≥3 to <6 months, and at 6-month periods thereafter. Three definitions of response in the absence of rescue therapy (composite of new cITP medication, increased dose of cITP medication from BL, platelet transfusion, and splenectomy) were considered: 1) platelet count ≥30 Gi/L (109/Liter); 2) platelet count ≥50 Gi/L; and 3) platelet count ≥50 Gi/L and > 2 times BL. Generalized estimating equations were used to assess the association between HRQoL over time and clinical response, accounting for within-patient correlation. Covariates included demographic characteristics and BL clinical characteristics (BMI, BL use of cITP medication, prior splenectomy, prior eltrombopag use, and BL platelet count).
Results:
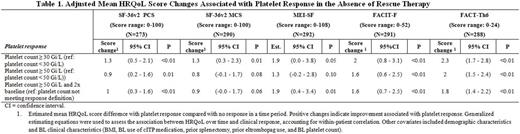
302 patients were enrolled in EXTEND. Between 273 and 292 (depending on instrument) had a BL, at least one on-treatment HRQoL assessment, and platelet count information. Baseline platelet count was <30 Gi/L for 69.9% of patients. Median treatment duration was 2.4 years. During treatment, 86.8% of the patients achieved platelet response of platelet count ≥30 Gi/L, 79.8% achieved ≥50 Gi/L, and 75.2% achieved ≥50 Gi/L and 2x BL at least once during the study. All HRQoL instruments had a positive best mean change from BL greater than a minimally important difference of at least half a standard deviation from BL score or an established threshold. All four HRQoL instruments had positive association with the three platelet response definitions. The adjusted mean score increase (signifying improvement) associated with platelet response compared to no response was 0.9-1.3 for SF-36v2 PCS, 0.8-1.3 for SF-36v2 MCS, 1.3-1.9 for MEI-SF, 1.6-2.0 for FACIT-F, and 1.8-2.3 for FACT-Th6 (most p <0.05; Table 1).
Conclusion:
These data show positive associations between platelet response and HRQoL measurements, especially with SF-36v2 PCS, FACIT-F, and FACT-Th6. Benefits from eltrombopag therapy in increasing platelet counts may carry forward into alleviating fatigue, concerns about bleeding and bruising, as well as enhancing motivation, energy, general physical and mental health status.
Saleh:GSK: Consultancy, Research Funding, Speakers Bureau. Bussel:Boehringer Ingelheim: Research Funding; Protalex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Physicians Education Resource: Speakers Bureau; Rigel Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prophylix Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; UpToDate: Patents & Royalties; Momenta Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Symphogen: Membership on an entity's Board of Directors or advisory committees; Shionogi: Membership on an entity's Board of Directors or advisory committees; Sysmex: Research Funding; Immunomedics: Research Funding; Genzyme: Research Funding; Cangene: Research Funding; BiologicTx: Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Burgess:Novartis: Employment. El-Ali:Novartis: Employment. Roy:Novartis: Employment. Barghout:Novartis: Consultancy. Duh:Novartis: Research Funding; Abbvie: Consultancy; Allergan: Consultancy; GSK: Research Funding; Bayer: Research Funding; Janssen: Research Funding; Eisai: Research Funding; Pfizer: Research Funding; Medtronic: Research Funding; Takeda: Research Funding; Novo Nordisk: Research Funding; Sanofi: Research Funding; Ariad: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.