Abstract
Introduction: Approximately 30% of patients with immune thrombocytopenia (ITP) fail to respond to the first- and/or second-line treatments (corticosteroids, intravenous immunoglobulin, rituximab, splenectomy, and thrombopoietin-receptor agonists). For those patients, the management is challenging.
Decitabine (DAC), a demethylating agent with a dual mechanism of action: the demethylating effect leading to cell differentiation at low dose and cytotoxic activity leading to cell death at high concentration, has been used in the management of myelodysplastic syndrome (MDS) with a considerable platelet response during the past decade. Recent studies proved that low-dose DAC was sufficient to show a therapeutic effect with no obvious cytotoxicity. Our previous and other studies have demonstrated that low-dose DAC could promote megakaryocyte maturation and platelet production. These findings suggest a possible therapeutic role of low-dose DAC in the management of ITP. We hereby present the preliminary results of a prospective, multicenter, open-labeled study evaluating the efficacy and safety of low-dose DAC for ITP patients.
Methods: ITP patients, who failed to respond to corticosteroids, intravenous immunoglobulin, rituximab, and/or thrombopoietin-receptor agonists from 9 centers, were enrolled in the study. The study protocol was approved by the ethics committee on medical research of each participating site. All patients provided written informed consent in accordance with the Declaration of Helsinki. DAC was given intravenously at 3.5mg/m2 for 3 days/cycle for 3 cycles with a 4-week interval between cycles.
The primary end points were complete response (CR), response (R), overall response (OR). All the criteria were consistent with the standardization of terminology, definitions and outcome criteria in immune thrombocytopenia proposed by the international working group (RodeghieroF, et al. Blood, 2009, 113:2386-2393). Secondary end points were bleeding scores, time to response (TTR), duration of response and adverse events. Adverse events were evaluated according to Common Terminology Criteria for Adverse Events, version3.0. This clinical trial was registered at http://clinicaltrials.gov as NCT 01568333.
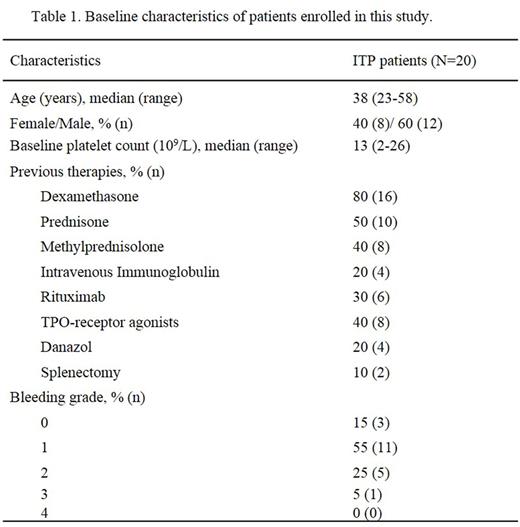
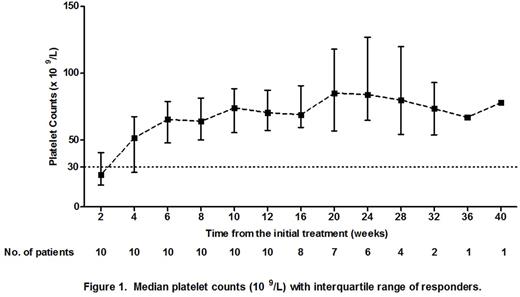
Results: A total of 20 ITP patients were recruited. The clinical characteristics were shown in Table 1. At the end of the 12th week of the initial treatment, CR was achieved in 1 patient (5%) and R was achieved in 9 patients (45%). The OR rate was 50%. During the follow-up period, 1 patient initially stabilized at R and subsequently improved to CR at the 20th week. Therefore, CR, R and OR rates were 10% (2/20), 40% (8/20) and 50% (10/20), respectively. In patients who achieved CR and R, the median (range) TTR was 22 days (8-38 days). The median (range) follow-up time was 24 weeks (13-40 weeks). The platelet counts of patients who achieved CR and R were shown in Figure 1. The follow-up of our study is in progress.
Adverse events were observed in 2 patients, one had nausea and the other was mild fever. No adverse events exceeded grade 1.
Conclusion: Although the sample size is small, with a relatively short follow-up period limited by now, our study suggests that low-dose DAC is effective and safe in the management of ITP patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.