Introduction
Romiplostim is a fusion protein agonist of the thrombopoietin receptor, approved by the US FDA for the treatment of immune thrombocytopenia (ITP) after failure of glucocorticoid therapy. This agent has been examined in other settings, including its use in the management of the thrombocytopenic surgical patient (Marshall et al., Transfusion 2015) and its use for the treatment of refractory aplastic anemia (Gill et al., Br J Haematol 2016). Thrombocytopenia is a complication of chemotherapy in cancer patients that may lead to treatment delays, dose reductions, and discontinuation of therapy (Kuter DJ, Oncology 2015). This study is a retrospective, single-center review of cancer patients who either developed thrombocytopenia as a result of chemotherapy or who had an underlying pre-existing thrombocytopenic disorder (such as ITP or chronic liver disease) who were supported with romiplostim during all or part of their chemotherapy.
Methods
We performed a retrospective review of patients with solid tumor malignancies who received at least two sequential weekly doses of romiplostim concurrently with chemotherapy. We collected patient demographics, dates and doses of romiplostim administration with corresponding platelet (Plt) counts, and outcomes of therapy, including ability of the treating oncologist to administer chemotherapy before initiating and during use of romiplostim as measured by number of treatment cycles administered, number and duration of delays in therapy, and number and extent of clinically necessary dose reductions.
Results
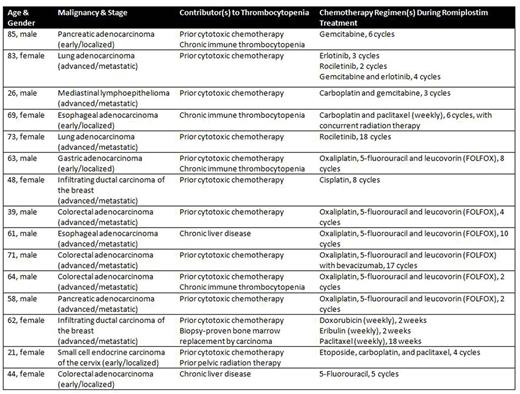
Review of pharmaceutical dispensation records from at our institution from January 1, 2010 through April 1, 2016 revealed 19 patients concurrently treated with romiplostim and chemotherapy, with 15 meeting our inclusion criteria (Table 1). At initiation of romiplostim, 40% of patients had a Plt count ≥75 x 109/L and 26.7% of patients had a Plt count ≥100 x 109/L, with a median Plt count of 72 x 109/L (range, 21-145 x 109/L). Patients were treated with weekly injections of romiplostim, with platelet count measured at the time of drug administration; the most common starting dose was 3 mcg/kg. The average duration of romiplostim therapy required to achieve a platelet count ≥75 x 109/L was 13.6 days, and the average time to achieve a platelet count ≥100 x 109/L was 21.1 days. During treatment with romiplostim, 85.3% and 73.2% of all platelet counts measured were ≥75 x 109/L and ≥100 x 109/L, respectively (Figure 1). All patients were able to receive two or more cycles (range, 2-18) of chemotherapy while on romiplostim. Twelve patients (80%) had delays in receiving antineoplastic therapy attributed to thrombocytopenia prior to initiation of romiplostim, with an average per-patient cumulative delay time of 4.7 weeks; 5 patients (33.3%) concurrently treated with romiplostim and chemotherapy had such delays (p=0.010 by Pearson's chi-squared test), with an average per-patient cumulative delay time of 1 week. Eleven patients (73.3%) required at least 1 dose-reduction in chemotherapy attributed to thrombocytopenia prior to initiation of romiplostim; 4 patients (26.7%) required at least 1 such dose-reduction while receiving romiplostim (p=0.011 by Pearson's chi-squared test). Three patients required platelet transfusion and 10 patients required red blood cell (RBC) transfusion while receiving romiplostim, though 8 of the patients received RBC transfusions only for chemotherapy-associated anemia or fatigue. While receiving romiplostim, no patient suffered a thrombotic event, and 3 patients suffered from bleeding (1 patient developed grade 1 mucosal bleeding and 2 patients developed grade 3 gastrointestinal bleeding).
Conclusion
In patients with solid tumor malignancies with chemotherapy-associated thrombocytopenia or who have co-existing thrombocytopenic disorders (such as ITP or chronic liver disease), this study provides evidence that romiplostim is safe and increases the platelet count sufficiently in a majority of patients to allow subsequent chemotherapy to be given at full dose and on schedule.
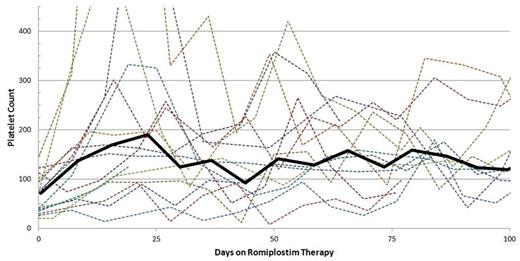
Platelet Count Over Time. The dotted lines represent the platelet count trend of individual patients. The solid black line represents the platelet count trend of the study population (platelet counts averaged together over seven-day intervals).
Platelet Count Over Time. The dotted lines represent the platelet count trend of individual patients. The solid black line represents the platelet count trend of the study population (platelet counts averaged together over seven-day intervals).
Kuter:Protalex: Research Funding; ONO: Consultancy; Pfizer: Consultancy; 3SBios: Consultancy; Eisai: Consultancy; GlaxoSmithKline: Consultancy; Bristol-Myers Squibb: Research Funding; Genzyme: Consultancy; Shire: Consultancy; Amgen: Consultancy, Paid expert testimony; Shionogi: Consultancy; Rigel: Consultancy, Research Funding; Syntimmune: Consultancy; MedImmune: Consultancy; CRICO: Other: Paid expert testimony.
Author notes
Asterisk with author names denotes non-ASH members.