Abstract
Background: Multiple myeloma (MM) is incurable by standard approaches, with relapse and development of treatment resistance inevitable in all patients. We previously identified a panel of novel macropinocytosing human monoclonal antibodies against CD46 by phage antibody library display and optimized a lead antibody for targeted drug delivery. Antibody-drug conjugates (ADCs) have recently seen proof-of-concept clinical success in Hodgkin lymphoma and breast cancer, but none is yet FDA-approved for MM. The CD46 gene is located on the long arm of chromosome 1 (1q32.2), 50Mbp from a FISH probe clinically used to identify high-risk MM and which may provide a surrogate biomarker for CD46 as a therapeutic target.
Methods:We covalently conjugated the monomethyl auristatin F (MMAF) toxin to our anti-CD46 antibody via a lysosomal protease sensitive valine-citrulline linker (hereafter referred to as CD46-ADC). High Performance Liquid Chromatography analysis with hydrophobic interaction chromatography of the final conjugate showed an average drug per antibody of 3.3. CD46-ADC was evaluated for cytotoxicity in vitro in MM cell lines, in vivo with cell line xenografts in NSG mice, and ex vivo in MM patient bone marrow (BM)aspirate samples. To assess in vivo toxicity, CD46-ADC treatment was administered to transgenic mice that express the human CD46 gene under its native promoter.
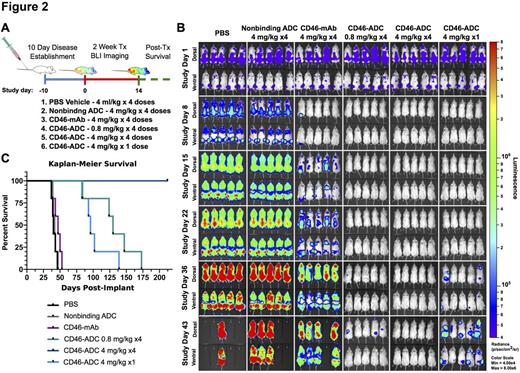
Results: CD46 was highly expressed on the cell surface of all 18 MM cell lines tested, and was upregulated on MM1.S cells co-cultured with the BM stromal cell line HS5. In BM aspirate samples, CD46 was highly expressed on MM cells in 100% (n=25) patients evaluated. By quantitative flow cytometry in 10 patients, the CD46 cell surface antigen density was significantly higher in patient MM cells with 1q21 gain (1q+) than those with normal 1q21 copy number (p=0.032) (Fig 1A). In patients with amp1q21 the mean CD46 antigen density on MM cells was 313,190 (SEM 68,849), compared to patients with normal 1q21 where it was 121,316 (SEM 28,352) (Fig 1A). In contrast, CD46 antigen density on normal donor (n=3) BM hematopoietic cell populations was low (antigen density range 8,443 - 23,772). Of note, higher CD46 antigen density was present on monocytes (mean 58,320, SEM 6,874) and granulocytes (mean 54,439, SEM 10,688) relative to the other populations (Fig 1B). CD46-ADC potently inhibited proliferation in all 14 MM cell lines tested (EC50 range of 150 pM - 5 nM) (Fig 1C). On BM stromal cells, CD46-ADC had EC50 >100 nM for patient-derived BM61 (generated via culture of CD138-negative BM) cells and no effect on HS5 cells in concentrations tested up to 150 nM. CD46-ADC eliminated MM growth in two orthometastatic xenograft models. In one model, MM1.S cell line xenografts expressing firefly luciferase grown in NSG mice were treated once every 3-4 days at either 4 mg/kg or 0.8 mg/kg for 4 injections, or with a single dose of 4 mg/kg (Fig 2A). Control groups were treated with vehicle, nonbinding ADC or naked antibody (CD46-mAb). CD46-ADC 4 mg/kg (4 dose) eliminated bioluminescent activity throughout the duration of the study (Fig 2B), and all mice survived to study discontinuation (Fig 2C). The single dose and low dose groups showed elimination of bioluminescence, but all mice relapsed (Fig 2B-C). In patient BM aspirate samples, CD46-ADC induces apoptosis and cell death in primary MM cells ex vivo (EC50 <10 nM), but did not affect the viability of non-tumor mononuclear cells (MNCs). For in vivo toxicity study, human CD46 transgenic mice were treated with a single IV bolus injection of 6 mg/kg CD46-ADC and showed no body weight loss or overt side effects for 14 days. At study discontinuation (day 14), histologic analysis of major organs showed no notable tissue damage.
Conclusion: We have identified a novel functional antigen, CD46, for ADC targeting of MM, with unique potential for high-risk and relapsed/refractory disease that has genomic amplification at the CD46 gene locus and are in dire need of therapy. The novel CD46-ADC is highly potent and selective in eliminating MM cells (cell lines and primary tumor cells) in preclinical models. CD46 genomic gain on chromosome 1q correlates with antigen amplification, andindentifies a potential biomarker based on a clinical FISH test that can be used for patient stratification. Thus, our study could lead directly to the application of a novel ADC therapeutic for treating MM.
Aftab:Onyx Pharmaceuticals, Inc.: Research Funding; Atara Biotherapeutics, Inc.: Employment, Equity Ownership; Omniox, Inc.: Research Funding; CytomX: Research Funding; Cleave Biosciences, Inc.: Research Funding. Wiita:Onyx Pharmaceuticals: Research Funding; Omniox, LLC: Research Funding; Cleave Biosciences: Research Funding; Quadriga Biosciences: Research Funding. Wolf:Celgene: Honoraria; Telomere Diagnostics: Consultancy; Takeda: Honoraria; Amgen: Honoraria; Pharmacyclics: Honoraria. Martin:Sanofi: Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.