Abstract
Background: Symptomatic venous thrombotic events (sVTE) are a well-recognized complication in pediatric oncology patients. Although data exists on incidence, risk factors and outcome of sVTE, the association of sVTE with survival outcome in pediatric oncology patients has not been previously assessed. The present study was designed to assess the survival outcome in pediatric oncology patients who developed sVTE as compared to those who did not develop sVTE.
Aims: To assess the overall survival (OS) and event free survival (EFS) in pediatric oncology patients with and without sVTE.
Methods: The Maritimes constitutes the 3 Canadian Provinces of Nova Scotia, New Brunswick and Prince Edward Island (total population = 1.8 million). All pediatric oncology patients from the Maritimes are treated at IWK Health Centre (IWK) in a shared care model with regional provincial hospitals. This provides a population-based cohort. Institutional ethics board approval for this retrospective study was obtained.
A database of all pediatric oncology patients treated at the IWK Health Centre from 2000-2015 was created by amalgamating data from (i) pediatric oncology hospital database (ii) Provincial Cancer in Young People registry (iii) pharmacy database and (iv) hospital health records. All pediatric oncology patients who developed sVTE were identified using the above databases. Data from all databases was cross-verified for accuracy and then pooled.
Data on patient demographics, diagnosis, date of diagnosis, date of last follow up, date of first relapse, and date of death were extracted. Status of every patient on or up to 31 December 2015 was reviewed.
SPSS version 22 was used for statistical analysis. Survival outcome was assessed using the Kaplan Meier method. Survival was reported in complete months.
sVTE was defined as radiologically documented VTE with at least one sign/symptom directly associated with VTE. First relapse or death were classified as events for calculation of EFS. Both OS and EFS were calculated from diagnosis of cancer.
Results: Forty-seven (5.02±0.01%) of the 936 patients had sVTE. The mean age at diagnosis for sVTE patients was 10.1 years. The gender ratio was male:female= 1.8:1 in patients with sVTE. Central veins were the most common location for sVTE (72.3%, n=34). The underlying cancer diagnosis in patients with sVTE were leukemia (36.2%, n=17), lymphoma (19.1%, n=9), sarcoma (19.1%, n=9), brain tumor (2.1%, n=1) and others (23.4%,n=11).
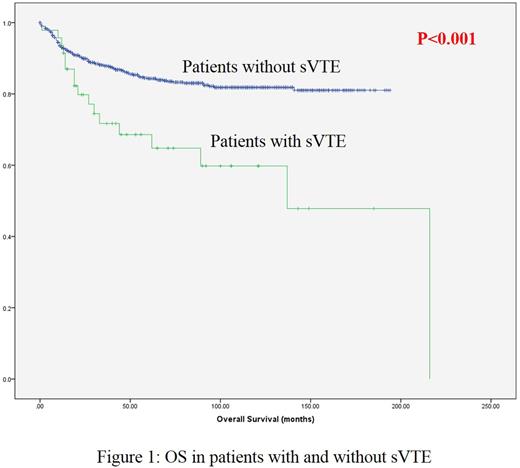
The OS of patients with sVTE was significantly inferior (p<0.001 by log rank test) as compared to those without sVTE (Figure 1). The estimated mean OS of patients with and without sVTE were 133.1 ±33.6 and 163.7 ±4.8 months respectively (p<0.001). The estimated 5 year OS for patients who developed sVTE was significantly inferior (p=0.007 by log rank test) as compared to those without VTE.
Of the 47 patients with sVTE, 17 (36.2%) died. In comparison, among patients without sVTE, 133 (15%) died (p=0.001).
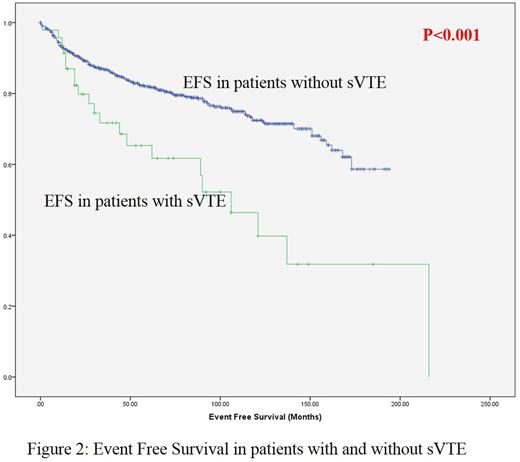
The EFS of patients with sVTE was significantly inferior (p<0.001 by log rank test) as compared those without sVTE (Figure 2). The estimated mean EFS of patients with and without sVTE were 112.7 ±31 and 148.7 ±5.9 months respectively (p<0.001).
Conclusion: In a large population-based cohort, we demonstrate that the survival outcome of pediatric oncology patients with sVTE is inferior as compared to those without sVTE. A plausible explanation is that patients at higher risk for death: poor prognosis/advanced cancers and those with more intense multimodality therapy are more likely to be diagnosed with sVTE. Validation of these findings is necessary, as is a better understanding of the drivers for this association of sVTE with early death and the possible role of thromboprophylaxis in optimizing outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.